A New Species of Poison-Dart Frog (Anura: Dendrobatidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

AC26 Doc. 20 Annex 1 English Only / Únicamente En Inglés / Seulement En Anglais
AC26 Doc. 20 Annex 1 English only / únicamente en inglés / seulement en anglais Fauna: new species and other taxonomic changes relating to species listed in the EC wildlife trade regulations January, 2012 A report to the European Commission Directorate General E - Environment ENV.E.2. – Environmental Agreements and Trade by the United Nations Environment Programme World Conservation Monitoring Centre AC26 Doc. 20, Annex 1 UNEP World Conservation Monitoring Centre 219 Huntingdon Road Cambridge CB3 0DL United Kingdom Tel: +44 (0) 1223 277314 Fax: +44 (0) 1223 277136 Email: [email protected] Website: www.unep-wcmc.org CITATION ABOUT UNEP-WORLD CONSERVATION UNEP-WCMC. 2012. Fauna: new species and MONITORING CENTRE other taxonomic changes relating to species The UNEP World Conservation Monitoring listed in the EC wildlife trade regulations. A Centre (UNEP-WCMC), based in Cambridge, report to the European Commission. UNEP- UK, is the specialist biodiversity information WCMC, Cambridge. and assessment centre of the United Nations Environment Programme (UNEP), run PREPARED FOR cooperatively with WCMC, a UK charity. The Centre's mission is to evaluate and highlight The European Commission, Brussels, Belgium the many values of biodiversity and put authoritative biodiversity knowledge at the DISCLAIMER centre of decision-making. Through the analysis and synthesis of global biodiversity The contents of this report do not necessarily knowledge the Centre provides authoritative, reflect the views or policies of UNEP or strategic and timely information for contributory organisations. The designations conventions, countries and organisations to use employed and the presentations do not imply in the development and implementation of the expressions of any opinion whatsoever on their policies and decisions. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Etar a Área De Distribuição Geográfica De Anfíbios Na Amazônia
Universidade Federal do Amapá Pró-Reitoria de Pesquisa e Pós-Graduação Programa de Pós-Graduação em Biodiversidade Tropical Mestrado e Doutorado UNIFAP / EMBRAPA-AP / IEPA / CI-Brasil YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRE LÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA Dissertação apresentada ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBIO) da Universidade Federal do Amapá, como requisito parcial à obtenção do título de Mestre em Biodiversidade Tropical. Orientador: Dra. Fernanda Michalski Co-Orientador: Dr. Rafael Loyola MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA _________________________________________ Dra. Fernanda Michalski Universidade Federal do Amapá (UNIFAP) _________________________________________ Dr. Rafael Loyola Universidade Federal de Goiás (UFG) ____________________________________________ Alexandro Cezar Florentino Universidade Federal do Amapá (UNIFAP) ____________________________________________ Admilson Moreira Torres Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) Aprovada em de de , Macapá, AP, Brasil À minha família, meus amigos, meu amor e ao meu pequeno Sebastião. AGRADECIMENTOS Agradeço a CAPES pela conceção de uma bolsa durante os dois anos de mestrado, ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBio) pelo apoio logístico durante a pesquisa realizada. Obrigado aos professores do PPGBio por todo o conhecimento compartilhado. Agradeço aos Doutores, membros da banca avaliadora, pelas críticas e contribuições construtivas ao trabalho. -

Cop18 Doc. 62 (Rev
Original language: Spanish CoP18 Doc. 62 (Rev. 1) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 Species specific matters DRAFT DECISIONS ON THE CONSERVATION OF AMPHIBIANS (AMPHIBIA) 1. This document has been submitted by Costa Rica.* 2. Amphibians are the most threatened class of vertebrates worldwide. According to the IUCN Red List of Threatened Species, "of the 6260 amphibian species assessed, nearly one-third of species (32.4 %) are globally threatened or extinct, representing 2030 species". This is considerably higher than the comparable figure for birds (13 %) or mammals (22 %) (IUCN Red List 2016). The number of species classified as Critically Endangered, Endangered, or Vulnerable has increased throughout the world due to a number of threats, including habitat loss, trade, and disease. While loss of habitat and disease are considered to be the major threats to amphibian populations, harvesting for human use is a further pressure, sometimes posing the greatest threat (IUCN, 2016). 3. Amphibians occur throughout the world, with diversity nuclei in Central America, South America, East and Central Africa, East and South Asia, and Madagascar (Abraham et al. 2013; Jenkins et al. 2013; Pratihar et al. 2014; Pimm et al. 2014, among other authors). Amphibians live in a variety of terrestrial and freshwater ecosystems, ranging from tropical forests to deserts (Stuart et al. 2008). 4. Amphibians have been an important part of human culture for millennia and traditionally served as a source of food (e.g., Mohneke et al. -

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
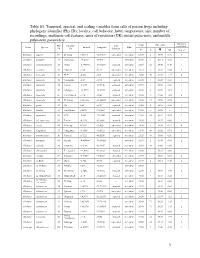
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Reproductive Biology of Ameerega Trivittata(Anura: Dendrobatidae)
ACTA AMAZONICA http://dx.doi.org/10.1590/1809-4392201305384 Reproductive biology of Ameerega trivittata (Anura: Dendrobatidae) in an area of terra firme forest in eastern Amazonia Ellen Cristina Serrão ACIOLI1 * , Selvino NECKEL-OLIVEIRA2 ¹ Universidade Federal do Pará/Museu Paraense Emílio Goeldi, Programa de Pós Graduação em Zoologia, Av. Perimetral 1901/1907, Terra Firme, 66017-970, Belém, Pará, Brasil. ² Universidade Federal de Santa Catarina, Departamento de Ecologia e Zoologia, Centro de Ciências Biológicas, 88040-970, Florianópolis, Santa Catarina, Brasil. * Corresponding author: [email protected] ABSTRACT The reproductive success of tropical amphibians is influenced by factors such as body size and the characteristics of breeding sites. Data on reproductive biology are important for the understanding of population dynamics and the maintenance of species. The objectives of the present study were to examine the abundance ofAmeerega trivittata, analyze the use of microhabitats by calling males and the snout-vent length (SVL) of breeding males and females, the number of tadpoles carried by the males and mature oocytes in the females, as well as the relationship between the SVL of the female and both the number and mean size of the mature oocytes found in the ovaries. Three field trips were conducted between January and September, 2009. A total of 31 plots, with a mean area of 2.3 ha, were surveyed, resulting in records of 235 individuals, with a mean density of 3.26 individuals per hectare. Overall, 66.1% of the individuals sighted were located in the leaf litter, while 17.4% were perched on decaying tree trunks on the forest floor, 15.7% on the aerial roots ofCecropia trees, and 0.8% on lianas. -

Diet Composition of Ameerega Picta
Bonn zoological Bulletin 68 (1): 93–96 ISSN 2190–7307 2019 · Landgref Filho P. et al. http://www.zoologicalbulletin.de https://doi.org/10.20363/BZB-2019.68.1.093 Scientific note urn:lsid:zoobank.org:pub:CBA156F4-E9AA-401C-B036-77C362CE1E89 Diet composition of Ameerega picta (Tschudi, 1838) from the Serra da Bodoquena region in central Brazil, with a summary of dietary studies on species of the genus Ameerega (Anura: Dendrobatidae) Paulo Landgref Filho1, Fabrício H. Oda2, *, Fabio T. Mise3, Domingos de J. Rodrigues4 & Masao Uetanabaro5 1 Universidade Federal de Mato Grosso do Sul, Campus Aquidauana, 79200-000, Aquidauana, Mato Grosso do Sul, Brazil 2 Departamento de Química Biológica, Programa de Pós-graduação em Bioprospecção Molecular, Universidade Regional do Cariri, 63105-00, Crato, Ceará, Brazil 3 Departamento de Biologia, Universidade Estadual do Centro-Oeste, 85040-080, Guarapuava, Paraná, Brazil 4 Instituto de Ciências Naturais, Humanas e Sociais, Universidade Federal de Mato Grosso – Campus Universitário de Sinop, 78557-267, Sinop, Mato Grosso, Brazil 4 Instituto Nacional de Ciência e Tecnologia de Estudos Integrados da Biodiversidade Amazônica – Núcleo Regional de Sinop, Sinop, Mato Grosso, Brazil 5 Rua Clóvis n. 24, 79022-071, Campo Grande, Mato Grosso do Sul, Brazil * Corresponding author: Email: [email protected] 1 urn:lsid:zoobank.org:author:5821C96D-4D9E-4E1D-BE9B-C231B77D1AF3 2 urn:lsid:zoobank.org:author:349712B5-E1B2-4059-A826-58F081DD3A4D 3 urn:lsid:zoobank.org:author:E996AF2A-6CB7-4B73-8DEF-AB7C5EEE1AA0 4 urn:lsid:zoobank.org:author:ACFBA432-3220-4910-AFF5-8FF85053A872 5 urn:lsid:zoobank.org:author:626F6AE3-7D90-4500-BFDC-4E6F4E01B378 Abstract. -

Species Diversity and Conservation Status Of
Florida International University FIU Digital Commons Department of Biological Sciences College of Arts, Sciences & Education 4-2009 Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru Rudolf Von May Department of Biological Sciences, Florida International University Karen Siu-Ting Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos Jennifer M. Jacobs San Francisco State University; California Academy of Sciences Margarita Medina-Muller Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos Giuseppe Gagliardi Museo de Zoología de la Universidad Nacional de la Amazonía Peruana See next page for additional authors Follow this and additional works at: https://digitalcommons.fiu.edu/cas_bio Part of the Biology Commons Recommended Citation May, Rudolf Von; Siu-Ting, Karen; Jacobs, Jennifer M.; Medina-Muller, Margarita; Gagliardi, Giuseppe; Rodriguez, Lily O.; and Donnelly, Maureen A., "Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru" (2009). Department of Biological Sciences. 164. https://digitalcommons.fiu.edu/cas_bio/164 This work is brought to you for free and open access by the College of Arts, Sciences & Education at FIU Digital Commons. It has been accepted for inclusion in Department of Biological Sciences by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. Authors Rudolf Von May, Karen Siu-Ting, Jennifer M. Jacobs, Margarita Medina-Muller, Giuseppe Gagliardi, Lily O. Rodriguez, and Maureen A. Donnelly This article is available at FIU Digital Commons: https://digitalcommons.fiu.edu/cas_bio/164 Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. -

Biodiversity Conservation and Phylogenetic Systematics Preserving Our Evolutionary Heritage in an Extinction Crisis Topics in Biodiversity and Conservation
Topics in Biodiversity and Conservation Roseli Pellens Philippe Grandcolas Editors Biodiversity Conservation and Phylogenetic Systematics Preserving our evolutionary heritage in an extinction crisis Topics in Biodiversity and Conservation Volume 14 More information about this series at http://www.springer.com/series/7488 Roseli Pellens • Philippe Grandcolas Editors Biodiversity Conservation and Phylogenetic Systematics Preserving our evolutionary heritage in an extinction crisis With the support of Labex BCDIV and ANR BIONEOCAL Editors Roseli Pellens Philippe Grandcolas Institut de Systématique, Evolution, Institut de Systématique, Evolution, Biodiversité, ISYEB – UMR 7205 Biodiversité, ISYEB – UMR 7205 CNRS MNHN UPMC EPHE, CNRS MNHN UPMC EPHE, Muséum National d’Histoire Naturelle Muséum National d’Histoire Naturelle Sorbonne Universités Sorbonne Universités Paris , France Paris , France ISSN 1875-1288 ISSN 1875-1296 (electronic) Topics in Biodiversity and Conservation ISBN 978-3-319-22460-2 ISBN 978-3-319-22461-9 (eBook) DOI 10.1007/978-3-319-22461-9 Library of Congress Control Number: 2015960738 Springer Cham Heidelberg New York Dordrecht London © The Editor(s) (if applicable) and The Author(s) 2016 . The book is published with open access at SpringerLink.com. Chapter 15 was created within the capacity of an US governmental employment. US copyright protection does not apply. Open Access This book is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. All commercial rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed.