DNA Barcoding for Identification of Anuran Species in the Central Region of South America
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Herpetological Journal FULL PAPER
Volume 26 (January 2017), 73–80 Herpetological Journal FULL PAPER Published by the British Herpetological Society Reproductive biology of the nest building vizcacheras frog Leptodactylus bufonius (Amphibia, Anura, Leptodactylidae), including a description of unusual courtship behaviour Gabriel Faggioni1, Franco Souza1, Masao Uetanabaro1, Paulo Landgref-Filho2, Joe Furman3 & Cynthia Prado1,4 1Programa de Pós-Graduação em Ecologia e Conservação, Universidade Federal de Mato Grosso do Sul, Campo Grande, Brasil 2Campo Grande, Brasil 3Houston, USA 4Departamento de Morfologia e Fisiologia Animal, Universidade Estadual Paulista, Jaboticabal, Brasil We describe the reproductive biology and sexual size dimorphism of a population of the vizcacheras frog Leptodactylus bufonius in the Brazilian Chaco. Reproduction takes place during the rainy months (September–March). During courtship, females emit reciprocal calls and both sexes perform vibratory movements of the body; the latter is described for the first time in anurans. Amplexus and oviposition occurred inside subterranean chambers. The temperature in closed chambers was lower than outside chambers, which may aid in reducing desiccation risks of eggs and tadpoles. Females were larger than males, but males had longer heads and shorter tibias, which may be related to digging. The study reinforces the importance of ongoing discoveries on anuran natural history. Keywords: Chaco, natural history, sexual size dimorphism, subterranean chamber, vibratory movements INTRODUCTION 1988; Haddad & Giaretta, 1999; Haddad & Sawaya, 2000; Lucas et al., 2008; Kokubum et al., 2009). he genus Leptodactylus Fitzinger, 1826, comprises 74 Species in the L. fuscus group reproduce in sub- species distributed from southern Texas to Argentina, terranean chambers which may vary in size, shape, includingT Caribbean islands (Frost, 2015). -

High Species Turnover Shapes Anuran Community Composition in Ponds Along an Urban-Rural Gradient
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.01.276378; this version posted September 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 High species turnover shapes anuran community composition in ponds along an urban-rural 2 gradient 3 4 Carolina Cunha Ganci1*, Diogo B. Provete2,3, Thomas Püttker4, David Lindenmayer5, 5 Mauricio Almeida-Gomes2 6 7 1 Pós-Graduação em Ecologia e Conservação, Universidade Federal de Mato Grosso do Sul, 8 Campo Grande, Mato Grosso do Sul, 79002-970, Brazil. 9 2 Instituto de Biociências, Universidade Federal de Mato Grosso do Sul, Campo Grande, Mato 10 Grosso do Sul, 79002-970, Brazil. 11 3 Göthenburg Global Biodiversity Centre, Göteborg, SE-450, Sweden. 12 4 Departamento de Ciências Ambientais, Universidade Federal de São Paulo - UNIFESP, São 13 Paulo, 09913-030, Brazil. 14 5 Fenner School of Environment and Societ, Australian National University, Canberra, ACT, 15 Australia. 16 17 * Corresponding author: [email protected] 18 19 Carolina Ganci orcid: 0000-0001-7594-8056 20 Diogo B. Provete orcid: 0000-0002-0097-0651 21 Thomas Püttker orcid: 0000-0003-0605-1442 22 Mauricio Almeida-Gomes orcid: 0000-0001-7938-354X 23 David Lindenmayer orcid: 0000-0002-4766-4088 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.01.276378; this version posted September 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Mudança Climática, Configuração Da Paisagem E Seus Efeitos Sobre a Fenologia E Biodiversidade De Anuros
i INSTITUTO FEDERAL DE EDUCAÇÃO, CIÊNCIA E TECNOLOGIA GOIANO - CAMPUS RIO VERDE PROGRAMA DE PÓS-GRADUAÇÃO BIODIVERSIDADE E CONSERVAÇÃO MUDANÇA CLIMÁTICA, CONFIGURAÇÃO DA PAISAGEM E SEUS EFEITOS SOBRE A FENOLOGIA E BIODIVERSIDADE DE ANUROS Autor: Seixas Rezende Oliveira Orientador: Dr. Matheus de Souza Lima Ribeiro Coorientador: Dr. Alessandro Ribeiro de Morais RIO VERDE – GO Fevereiro – 2018 ii INSTITUTO FEDERAL DE EDUCAÇÃO, CIÊNCIA E TECNOLOGIA GOIANO - CAMPUS RIO VERDE PROGRAMA DE PÓS- GRADUAÇÃO BIODIVERSIDADE E CONSERVAÇÃO MUDANÇA CLIMÁTICA, CONFIGURAÇÃO DA PAISAGEM E SEUS EFEITOS SOBRE A FENOLOGIA E BIODIVERSIDADE DE ANUROS Autor: Seixas Rezende Oliveira Orientador: Dr. Matheus de Souza Lima Ribeiro Coorientador: Dr. Alessandro Ribeiro de Morais Dissertação apresentada, como parte das exigências para obtenção do título de MESTRE EM BIODIVERSIDADE E CONSERVAÇÃO, no Programa de Pós- Graduação em Biodiversidade e conservação do Instituto Federal de Educação, Ciência e Tecnologia Goiano – Campus Rio Verde - Área de Concentração Conservação dos recursos naturais. RIO VERDE – GO Fevereiro – 2018 iii iv v DEDICO ESTE TRABALHO: Aos meus amados pais João Batista Oliveira Rezende e Rita Maria Rezende Oliveira. À meu irmão Fagner Rezende Oliveira e a meus sobrinhos Jorge Otavio Rezende Valdez e João Miguel Rezende Valdez. vi AGRADECIMENTOS A toda minha família, em especial Pai, Mãe e Irmão que nunca mediram esforços para que eu seguisse firme nos estudos, e proporcionaram a mim educação, um lar confortante e seguro, onde sempre busquei minhas forças e inspirações para seguir em frente com todos os projetos de vida. Ao meu orientador e amigo Prof. Dr. Matheus de Souza Lima Ribeiro, exemplo de pessoa em todos os quesitos, falta adjetivos que descreve tamanhas qualidades, que mesmo com muitos afazeres, sempre doou seu tempo para me ajudar sendo essencial para elaboração e condução deste trabalho. -

Diptera: Sarcophagidae) in Anuran of Leptodactylidae (Amphibia)
CASO CLÍNICO REVISTA COLOMBIANA DE CIENCIA ANIMAL Rev Colombiana Cienc Anim 2015; 7(2):217-220. FIRST REPORT OF MYIASIS (DIPTERA: SARCOPHAGIDAE) IN ANURAN OF LEPTODACTYLIDAE (AMPHIBIA) PRIMER REGISTRO DE MIASIS (DIPTERA: SARCOPHAGIDAE) EN ANUROS DE LEPTODACTYLIDAE (AMPHIBIA) GERSON AZULIM MÜLLER,1*Dr, CARLOS RODRIGO LEHN,1 M.Sc, ABEL BEMVENUTI,1 M.Sc, CARLOS BRISOLA MARCONDES,2 Dr. 1Instituto Federal de Educação, Ciência e Tecnologia Farroupilha, Campus Panambi, RS, Brasil. 2 Universidade Federal de Santa Catarina, Departamento de Microbiologia e Parasitologia, Centro de Ciências Biológicas, SC, Brasil. Key words: Abstract Anura, This note is the first report of myiasis caused by Sarcophagidae flies in an anuran of Brazil, Leptodactylidae. The frog, identified asLeptodactylus latrans (Steffen, 1815), was Leptodactylus latrans, collected in Atlantic forest bioma, southern Brazil. The frog had extensive muscle parasitism. damage and orifices in the tegument caused by presence of 21 larvae, identified as Sarcophagidae. Ecological interactions between dipterans and anuran are poorly known. The impact of sarcophagid flies in anuran popuilations requires further study. Palabras Clave: Resumen Anura, Esta nota es el primer registro de ocurrencia de miasis generada por moscas Brasil, Sarcophagidae en anuro de la familia Leptodactylidae. El anfibio, identificado Leptodactylus latrans, como Leptodactylus latrans (Steffen, 1815), fue recolectado en el bioma Mata parasitismo. Atlântica, en el sur de Brasil. La rana presentaba extensas lesiones musculares y orificios en el tegumento generados por la presencia de 21 larvas, identificadas como Sarcophagidae. Las interacciones ecológicas entre insectos dípteros y anuros son poco conocidas. El impacto de las moscas Sarcophagidae en las poblaciones de anuros requiere más estudio. -

Vocal Repertory of Two Species of the Leptodactylus Pentadactylus Group (Anura, Leptodactylidae)
ISSN 1094-2246 Contemporary Herpetology Volume 2008, Number 1 13 April 2008 contemporaryherpetology.org VOCAL REPERTORY OF TWO SPECIES OF THE LEPTODACTYLUS PENTADACTYLUS GROUP (ANURA, LEPTODACTYLIDAE) WAGNER RODRIGUES DA SILVA1, ARIOVALDO ANTÔNIO GIARETTA AND KÁTIA GOMES FACURE Laboratório de Taxonomia, Sistemática e Ecologia Comportamental de Anuros Neotropicais, Instituto de Biologia, Universidade Federal de Uberlândia, Uberlândia (MG), Brazil, CEP: 38 400-902. E-mail: thoro- [email protected] ABSTRACT: Among frogs, vocalizations play important roles in their social interactions. Herein we de- scribe fi ve new types of vocalizations for two foam-nesting species of the Leptodactylus pentadacty- lus group, L. syphax and L. labyrinthicus. Behavioral observations and recordings were done in four localities within the Cerrado biome, at southeast and central Brazil. Before emitting advertisement calls, males of L. syphax often started producing a sequence of notes, which gradually turned into the advertisement call. These different notes may be an introductory call, which would serve to prepare the vocal structures for the emission of the high-frequency/amplitude advertisement calls. A male of L. syphax was emitting advertisement calls when a female approached and started to emit brief and low-amplitude calls; these vocalizations probably are reciprocation calls. Males of L. labyrinthicus involved in agonistic interactions can emit vocal cracks (encounter call) and deep rough sounds (ter- ritorial calls). Five courting males of L. labyrinthicus released screams with their mouth slightly opened in response to the approach of human observers. We conclude that these screams do not represent distress or territorial calls. Key Words: Leptodactylus labyrinthicus, L. syphax, male vocalizations, female vocalization. -
For Review Only
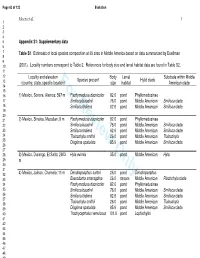
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Amphibians of an Open Cerrado Fragment in Southeastern Brazil
AMPHIBIANS OF AN OPEN CERRADO FRAGMENT IN SOUTHEASTERN BRAZIL Cínthia Aguirre Brasileiro1** , Ricardo J. Sawaya2, Mara C. Kiefer3, Marcio Martins 4 Biota Neotropica v5 (n2) – http://www.biotaneotropica.org.br/v5n2/pt/abstract?article+BN00405022005 Date Received 03/07/2005 Revised 05/06/2005 Accepted 07/01/2005 1Museu de História Natural, Instituto de Biologia, Universidade Estadual de Campinas, C. P. 6109, 13083-970 Campinas SP, and Departamento de Ecologia, Instituto de Biociências, Universidade de São Paulo, Rua do Matão, Trav. 14 s/n, 05508-090 São Paulo SP, Brasil. **Autor para correspondência: [email protected] 2Laboratório de Herpetologia, Instituto Butantan, Av. Dr. Vital Brazil 1500, 05503-900 São Paulo SP, Brasil 3Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, C. P. 6109, 13083-970 Campinas SP, Brasil, and Departamento de Ecologia, IBRAG, Universidade Estadual do Rio de Janeiro, Rua São Francisco Xavier 524, Maracanã, 20550-019 Rio de Janeiro, RJ, Brasil. 4Departamento de Ecologia, Instituto de Biociências, Universidade de São Paulo, Rua do Matão, Trav. 14 s/n, 05508-090 São Paulo SP, Brasil Abstract The Cerrado encompasses ca. 2 million km2 in Brazil. Most Cerrado areas have been greatly disturbed in the past decades. Only 20% of this biome remain undisturbed, and only 1.2% is protected. Knowledge on the biology and diversity of Cerrado amphibian assemblages is still incipient. Here we present natural history information (habitat use and reproductive activity) of 28 species of frogs from the Estação Ecológica de Itirapina (EEI), and compare the composition of this assem- blage with those of other Cerrado areas, other open areas, and a few forest areas throughout Brazil. -

Etar a Área De Distribuição Geográfica De Anfíbios Na Amazônia
Universidade Federal do Amapá Pró-Reitoria de Pesquisa e Pós-Graduação Programa de Pós-Graduação em Biodiversidade Tropical Mestrado e Doutorado UNIFAP / EMBRAPA-AP / IEPA / CI-Brasil YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRE LÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA Dissertação apresentada ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBIO) da Universidade Federal do Amapá, como requisito parcial à obtenção do título de Mestre em Biodiversidade Tropical. Orientador: Dra. Fernanda Michalski Co-Orientador: Dr. Rafael Loyola MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA _________________________________________ Dra. Fernanda Michalski Universidade Federal do Amapá (UNIFAP) _________________________________________ Dr. Rafael Loyola Universidade Federal de Goiás (UFG) ____________________________________________ Alexandro Cezar Florentino Universidade Federal do Amapá (UNIFAP) ____________________________________________ Admilson Moreira Torres Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) Aprovada em de de , Macapá, AP, Brasil À minha família, meus amigos, meu amor e ao meu pequeno Sebastião. AGRADECIMENTOS Agradeço a CAPES pela conceção de uma bolsa durante os dois anos de mestrado, ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBio) pelo apoio logístico durante a pesquisa realizada. Obrigado aos professores do PPGBio por todo o conhecimento compartilhado. Agradeço aos Doutores, membros da banca avaliadora, pelas críticas e contribuições construtivas ao trabalho. -

Dietary Resource Use by an Assemblage of Terrestrial Frogs from the Brazilian Cerrado
NORTH-WESTERN JOURNAL OF ZOOLOGY 15 (2): 135-146 ©NWJZ, Oradea, Romania, 2019 Article No.: e181502 http://biozoojournals.ro/nwjz/index.html Dietary resource use by an assemblage of terrestrial frogs from the Brazilian Cerrado Thiago MARQUES-PINTO1,*, André Felipe BARRETO-LIMA1,2,3 and Reuber Albuquerque BRANDÃO1 1. Laboratório de Fauna e Unidades de Conservação, Departamento de Engenharia Florestal, Universidade de Brasília, Brasília – DF, Brazil. 70.910-900, [email protected] 2. Departamento de Ciências Fisiológicas, Instituto de Ciências Biológicas, Universidade de Brasília, Campus Darcy Ribeiro, Brasília – DF, Brazil. 70.910-900, [email protected] 3. Programa de Pós-Graduação em Ecologia, Instituto de Ciências Biológicas, Universidade de Brasília, Campus Darcy Ribeiro, Brasília – DF, Brazil. 70.910-900. *Corresponding author, T. Marques-Pinto, E-mail: [email protected] Received: 08. June 2016 / Accepted: 07. April 2018 / Available online: 19. April 2018 / Printed: December 2019 Abstract. Diet is an important aspect of the ecological niche, and assemblages are often structured based on the ways food resources are partitioned among coexisting species. However, few works investigated the use of food resources in anuran communities in the Brazilian Cerrado biome. Thereby, we studied the feeding ecology of an anuran assembly composed of six terrestrial frog species in a Cerrado protected area. Our main purpose was to detect if there was a structure in the assemblage based on the species’ diet, in terms of the feeding niche overlap and the species’ size. All specimens were collected by pitfall traps placed along a lagoon margin, during the rainy season. We collected six frog species: Elachistocleis cesarii (172 individuals), Leptodactylus fuscus (10), L. -

Instituto De Biociências – Rio Claro Programa De Pós
UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” unesp INSTITUTO DE BIOCIÊNCIAS – RIO CLARO PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS (ZOOLOGIA) ANFÍBIOS DA SERRA DO MAR: DIVERSIDADE E BIOGEOGRAFIA LEO RAMOS MALAGOLI Tese apresentada ao Instituto de Biociências do Câmpus de Rio Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de doutor em Ciências Biológicas (Zoologia). Agosto - 2018 Leo Ramos Malagoli ANFÍBIOS DA SERRA DO MAR: DIVERSIDADE E BIOGEOGRAFIA Tese apresentada ao Instituto de Biociências do Câmpus de Rio Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de doutor em Ciências Biológicas (Zoologia). Orientador: Prof. Dr. Célio Fernando Baptista Haddad Co-orientador: Prof. Dr. Ricardo Jannini Sawaya Rio Claro 2018 574.9 Malagoli, Leo Ramos M236a Anfíbios da Serra do Mar : diversidade e biogeografia / Leo Ramos Malagoli. - Rio Claro, 2018 207 f. : il., figs., gráfs., tabs., fots., mapas Tese (doutorado) - Universidade Estadual Paulista, Instituto de Biociências de Rio Claro Orientador: Célio Fernando Baptista Haddad Coorientador: Ricardo Jannini Sawaya 1. Biogeografia. 2. Anuros. 3. Conservação. 4. Diversidade funcional. 5. Elementos bióticos. 6. Mata Atlântica. 7. Regionalização. I. Título. Ficha Catalográfica elaborada pela STATI - Biblioteca da UNESP Campus de Rio Claro/SP - Ana Paula Santulo C. de Medeiros / CRB 8/7336 “To do science is to search for repeated patterns, not simply to accumulate facts, and to do the science of geographical ecology is to search for patterns of plant and animal life that can be put on a map. The person best equipped to do this is the naturalist.” Geographical Ecology. Patterns in the Distribution of Species Robert H. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

AMPHIBIA: ANURA: LEPTODACTYLIDAE Leptodactylus Pentadactylus
887.1 AMPHIBIA: ANURA: LEPTODACTYLIDAE Leptodactylus pentadactylus Catalogue of American Amphibians and Reptiles. Heyer, M.M., W.R. Heyer, and R.O. de Sá. 2011. Leptodactylus pentadactylus . Leptodactylus pentadactylus (Laurenti) Smoky Jungle Frog Rana pentadactyla Laurenti 1768:32. Type-locality, “Indiis,” corrected to Suriname by Müller (1927: 276). Neotype, Nationaal Natuurhistorisch Mu- seum (RMNH) 29559, adult male, collector and date of collection unknown (examined by WRH). Rana gigas Spix 1824:25. Type-locality, “in locis palu - FIGURE 1. Leptodactylus pentadactylus , Brazil, Pará, Cacho- dosis fluminis Amazonum [Brazil]”. Holotype, Zoo- eira Juruá. Photograph courtesy of Laurie J. Vitt. logisches Sammlung des Bayerischen Staates (ZSM) 89/1921, now destroyed (Hoogmoed and Gruber 1983). See Nomenclatural History . Pre- lacustribus fluvii Amazonum [Brazil]”. Holotype, occupied by Rana gigas Wallbaum 1784 (= Rhin- ZSM 2502/0, now destroyed (Hoogmoed and ella marina {Linnaeus 1758}). Gruber 1983). Rana coriacea Spix 1824:29. Type-locality: “aquis Rana pachypus bilineata Mayer 1835:24. Type-local MAP . Distribution of Leptodactylus pentadactylus . The locality of the neotype is indicated by an open circle. A dot may rep - resent more than one site. Predicted distribution (dark-shaded) is modified from a BIOCLIM analysis. Published locality data used to generate the map should be considered as secondary sources, as we did not confirm identifications for all specimen localities. The locality coordinate data and sources are available on a spread sheet at http://learning.richmond.edu/ Leptodactylus. 887.2 FIGURE 2. Tadpole of Leptodactylus pentadactylus , USNM 576263, Brazil, Amazonas, Reserva Ducke. Scale bar = 5 mm. Type -locality, “Roque, Peru [06 o24’S, 76 o48’W].” Lectotype, Naturhistoriska Riksmuseet (NHMG) 497, age, sex, collector and date of collection un- known (not examined by authors).