FAU Institutional Repository
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Biology Research Biodiversity in Concert: Common, Uncommon
This article was downloaded by: On: 11 February 2010 Access details: Access Details: Free Access Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37- 41 M ortim er Street, London W lT 3JH, UK Marine Biology Research Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713735885 Marine Biology Research former# Santa tNrfQMMo Biodiversity in concert: Common, uncommon, and new species Tom Fenchel; Franz Uiblein Online publication date: 09 December 2009 To cite this ArticleFenchel, Tom and Uiblein, Franz(2010) 'Biodiversity in concert: Common, uncommon, and new species', Marine Biology Research, 6: 1, 1 — 5 To link to this Article: DOI:10.1080/17451000903468856 URL: http://dx.doi.org/10.1080/17451000903468856 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. -

Vampire Squid Final
An Examination regarding the Phylogenetic Position of Vampyroteuthis infernalis Printing: Alex Dutton, Chase Klungle, Jake Nymeyer This poster is 48” wide by 36” high. It’s designed to be printed on a INTRODUCTION METHODS RESULTS large Vampire squid: • Research included 43 in-class taxa with one additional in-class outgroup. • Vampyroteuthis infernalis, “the living fossil” is found nested in-between the Vampyroteuthis infernalis, or the Vampire Squid, is a cephalopod found deep in the • Cross examination of two genes was implemented (H3 and Ribosomal 28s genes). suborder Cirrata (Octopuses) and the Order Oegopsida (squid) while exhibiting characteristics of both. ocean. It has 8 arms connected by a webbing or “cape,” and is typically black in color • Individual gene processing was accomplished utilizing Phylogeny.fr wherein: with red eyes. These attributes led to it being called a vampire (not because it drinks alignment of data was processed by MUSCLE, curation by Gblocks, Phylogeny analysis • This data shows V. infernalis as being contained within the monophyletic Customizing the Content: blood). This species exhibits traits that appear in both octopus and squid families by PhyML + aLRT, and initial tree rendering by TreeDyn. supergroup Octopodiformes. which results in a one-of-a-kind organism. However, the phylogenetic position of V. • SequenceMatrix was employed to combine the aligned gene sequences. • We can also note the increased evolutionary distance between V. infernalis and infernalis has yet to be truly defined. Some researchers believe that it aligns better squids as opposed to their closeness in previous research. The placeholders in this with squids while others side with its closeness to octopuses. -
Highlights Report with Live Links
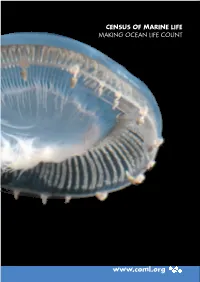
CENSUS OF MARINE LIFE MAKING OCEAN LIFE COUNT www.coml.org Census of Marine Life Projects Information System: OBIS Nearshore: NaGISA Coral Reefs: CReefs Regional Ecosystems: GoMA Continental Shelves: POST Continental Margins: COMARGE Abyssal Plains: CeDAMar Mid-Ocean Ridges: MAR-ECO Seamounts: CenSeam Vents and Seeps: ChEss Arctic Ocean: ArcOD Antarctic Ocean: CAML Top Predators: TOPP Zooplankton: CMarZ Microbes: ICoMM Oceans Past: HMAP Oceans Future: FMAP National and Regional Implementation Committees Australia Canada Caribbean China Europe Japan Indian Ocean Indonesia South Korea South America Sub-Saharan Africa United States Like an underwater spaceship, a jellyfish,Aequorea macrodactyla, travels through the warm, clear waters of the Celebes Sea in the Western Pacific Ocean. The jellyfish was but one of thousands of specimens photographed during a three-week Census expedition to explore this highly diverse area. Photo: Larry Madin, Woods Hole Oceanographic Institution. A Truly Global Endeavor The Census of Marine Life is a global network of researchers in more than 80 nations engaged in a ten-year scientific initiative to assess and ex- plain the diversity, distribution, and abundance of marine life in the oceans. The world’s first com- prehensive Census of Marine Life—past, present, and future—will be released in 2010. The Census gratefully acknowledges the financial support of numerous governments and organiza- tions from around the world. Moreover, many of the highlights in this report were only realized through the generous collaborative spirit and un- precedented cooperation of Census researchers and their international colleagues. A complete list of Census sponsors, funding partners, collaborat- ing institutions, and participating individuals is available at www.coml.org. -

The Phylogeny of Coleoid Cephalopods Inferred from Molecular Evolutionary Analyses of the Cytochrome C Oxidase I, Muscle Actin, and Cytoplasmic Actin Genes
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1998 The phylogeny of coleoid cephalopods inferred from molecular evolutionary analyses of the cytochrome c oxidase I, muscle actin, and cytoplasmic actin genes David Bruno Carlini College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Genetics Commons, Molecular Biology Commons, and the Zoology Commons Recommended Citation Carlini, David Bruno, "The phylogeny of coleoid cephalopods inferred from molecular evolutionary analyses of the cytochrome c oxidase I, muscle actin, and cytoplasmic actin genes" (1998). Dissertations, Theses, and Masters Projects. Paper 1539616597. https://dx.doi.org/doi:10.25773/v5-3pyk-f023 This Dissertation is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter free, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

Full Text in Pdf Format
Vol. 5: 13–22, 2009 AQUATIC BIOLOGY Printed March 2009 doi: 10.3354/ab00117 Aquat Biol Published online February 3, 2009 OPEN ACCESS Vertical distribution, behavior, chemical composition and metabolism of Stauroteuthis syrtensis (Octopoda: Cirrata) in the northwest Atlantic Charles A. Jacoby1,*, Marsh J. Youngbluth2, Jessica R. Frost1, 8, Per R. Flood3, Franz Uiblein4, Ulf Båmstedt5, Francesc Pagès6,†, David Shale7 1University of Florida, Gainesville, Florida 32653, USA 2Harbor Branch Oceanographic Institution at Florida Atlantic University, 5600 Highway 1 North, Fort Pierce, Florida 34946, USA 3Bathybiologica A/S, Gerhard Grans vei 58, 5081 Bergen, Norway 4Institute of Marine Research, Nordnesgaten 33, PB 1870 Nordnes, 5817 Bergen, Norway 5Umeå Marine Sciences Centre, Norrbyn, 910 20 Hörnefors, Sweden 6Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta 37–49, 08003 Barcelona, Spain 714 Victoria Avenue, Swanage, Dorset BH19 1AN, UK 8Present address: Institute for Hydrobiology and Fisheries Science, University of Hamburg, Olbersweg 24, 22767 Hamburg, Germany ABSTRACT: The cirrate octopod Stauroteuthis syrtensis is a mesopelagic species commonly collected in the North Atlantic. Individuals were observed at depths >600 m and typically within 100 m of the bot- tom in three ~900 m deep canyons indenting the southern edge of Georges Bank. When first sighted, most octopods were floating passively with their webbed arms gathered into a small ball. When dis- turbed, they expanded their webs to form a ‘balloon’ shape, swam slowly by sculling their fins, pulsed their webs like medusae and, in some cases, streamlined their arms and webs and moved away smoothly by rapidly sculling their fins. The bodies of 9 octopods comprised 92 to 95% water, with tissue containing 9 to 22% carbon (C) and 2 to 4% nitrogen (N). -

GUIDELINES for TAXONOMIC DESCRIPTIONS of CEPHALOPOD SPECIES by CLYDE F
a GUIDELINES FOR TAXONOMIC DESCRIPTIONS OF CEPHALOPOD SPECIES By CLYDE F. E. ROPER* AND GILBERT L. Vossf * Department of Invertebrate Zoology—Mollusks . ' National Museum of Natural History, Smithsonian Institution, Washington, DC, USA t Rosenstiel School of Marine and Atmospheric Science ] University of Miami, Miami, FL, USA Abstract .; This paper presents a format of guidelines considered necessary for the description (or redescription) of species of cephalopods. These guidelines or standards include specific requirements for descriptive characters of species within the Orders Sepioidea, Teuthoidea and Octopoda as well as general informa- tion, e.g., synonymy, locality, etc. Standards are given for descriptions, counts of measurements, and illustrations. Appendices list definitions of counts, measurements, and indices; diagramatically illustrate standard measurements; and give examples from the literature of descriptions that approach these standards. General Standards new zoological species (and other taxa). The Introduction. The need for minimum stan- naming of any new taxon should adhere to the dards for the description of species in rules and recommendations of the International cephalopod systematics was addressed at the Code of Zoological Nomenclature (Stoll et al., International Workshop on the Biology and 1964). Resource Potential of Cephalopods (Roper, The following criteria are considered 1983) sponsored by the National Museum of necessary to adequately describe a new species Victoria and the Victorian Institute of Marine or to redescribe a species inadequately des- , Sciences and held at the Marine Sciences cribed originally. Ideally, at least five specimens ! Laboratory, Queenscliff, Australia, 9-13 March consisting of both males and females should be ! 1981. We are most grateful to a number of the considered as a prerequisite for describing a workshop participants who responded to the new species. -

Stauroteuthis Syrtensis
New York City, 4 February 2011, MBL Board of Overseers Planet Ocean A Synthesis of the Census The Census of Marine Life: A Look at All Life in the Sea Jesse H. Ausubel Alfred P. Sloan Foundation and The Rockefeller University A reminder of features of Planet Ocean Why a Census: Wave energy machine (concept) A more crowded ocean and yet an unexplored, unknown ocean More uses, more users 3 Drilling derricks Crowding: Maritime Transport Time lapse tracks of vessels > 300 gross tons, 2009 Busy highways but also areas with few vessels; 3x # of large ships since 1960 Source: US Coast Guard4 Offshore oil & gas: Extensive, complex networks Gulf of Mexico pipeline network Source: NOIA 5 How a Census? People and technology Source: CoML NaGISA At work in Antarctic Waters Source: CoML CAML Polar Sampling How: A concerto of technologies What? The Census surveyed Diversity: Kinds of life Richer Distribution: Where they live & travel More connected Abundance: How much of each kind More altered Some newly discovered species: North Atlantic deep-sea octopod Stauroteuthis syrtensis Source: CoML MAR-ECO Celebes Sea, new “squidworm” Source: CoML CMarZ Sea cucumber Enypniastes caught at 2,750 meters on the continental margin in the Celebes Sea between Indonesia and the Philippines Source: CoML CMarZ Abyssal plains, S. Atlantic copepod Ceratonotus steiningeri Source: CoML CEDAMAR Source: Pedro Martínez Arbizu – CeDAMar Diversity never imagined Angola Basin: > 800 different copepods, most new to science Southern Ocean: > 700 different isopods, > 500 new to science 15 Source: Pedro Martínez Arbizu – CeDAMar Astounding discoveries, richness A group of carnivorous sponges abundant & species rich in the deep sea 16 © D. -

Sönke Johnsen
Sönke Johnsen Education: University of North Carolina at Chapel Hill: Ph.D., Biology, 1996, 1990- 1996 Dr. William M. Kier, advisor Swarthmore College: B.A. with Distinction, Mathematics, 1988, 1984-1988 Phi Beta Kappa and National Merit Scholarship Professional experience: Professor, Biology Department, Duke University, Durham, NC 2012- Research Associate, Smithsonian Museum of Natural History 2012-2018 Adjunct Professor, Nicholas School of the Environment, Duke University 2003- Associate Professor, Biology Department, Duke University 2007-2012 Assistant Professor, Biology Department, Duke University 2001-2007 Adjunct Scientist, Woods Hole Oceanographic Institution 2002-2005 Assistant Scientist, Woods Hole Oceanographic Institution 2000-2001 Postdoctoral Scholar, Woods Hole Oceanographic Institution, 1999-2000 Dr. Laurence P. Madin, advisor Postdoctoral Fellow, Harbor Branch Oceanographic Institution, 1997-1998 Dr. Edith A. Widder, advisor Lecturer, Department of Biology, University of North Carolina at Chapel Hill 1996-1997 National Science Foundation Pre-Doctoral Fellow, Department of Biology, 1991-1994 University of North Carolina at Chapel Hill Awards, honors, and fellowships: Deans Award for Excellence in Mentoring, Duke University 2016 Hilgendorf Lecturer, University of Tübingen, Germany 2014 Miller Institute Symposium Speaker, Miller Institute, Berkeley, CA 2014 Paul Illg Distinguished Lecturer, Friday Harbor Laboratories 2010 Schmidt-Nielsen Memorial Lecturer, Duke University 2010 University Distinguished Teaching Award, Duke -

Cephalopods As Predators: a Short Journey Among Behavioral Flexibilities, Adaptions, and Feeding Habits
REVIEW published: 17 August 2017 doi: 10.3389/fphys.2017.00598 Cephalopods as Predators: A Short Journey among Behavioral Flexibilities, Adaptions, and Feeding Habits Roger Villanueva 1*, Valentina Perricone 2 and Graziano Fiorito 3 1 Institut de Ciències del Mar, Consejo Superior de Investigaciones Científicas (CSIC), Barcelona, Spain, 2 Association for Cephalopod Research (CephRes), Napoli, Italy, 3 Department of Biology and Evolution of Marine Organisms, Stazione Zoologica Anton Dohrn, Napoli, Italy The diversity of cephalopod species and the differences in morphology and the habitats in which they live, illustrates the ability of this class of molluscs to adapt to all marine environments, demonstrating a wide spectrum of patterns to search, detect, select, capture, handle, and kill prey. Photo-, mechano-, and chemoreceptors provide tools for the acquisition of information about their potential preys. The use of vision to detect prey and high attack speed seem to be a predominant pattern in cephalopod species distributed in the photic zone, whereas in the deep-sea, the development of Edited by: Eduardo Almansa, mechanoreceptor structures and the presence of long and filamentous arms are more Instituto Español de Oceanografía abundant. Ambushing, luring, stalking and pursuit, speculative hunting and hunting in (IEO), Spain disguise, among others are known modes of hunting in cephalopods. Cannibalism and Reviewed by: Francisco Javier Rocha, scavenger behavior is also known for some species and the development of current University of Vigo, Spain culture techniques offer evidence of their ability to feed on inert and artificial foods. Alvaro Roura, Feeding requirements and prey choice change throughout development and in some Institute of Marine Research, Consejo Superior de Investigaciones Científicas species, strong ontogenetic changes in body form seem associated with changes in (CSIC), Spain their diet and feeding strategies, although this is poorly understood in planktonic and *Correspondence: larval stages. -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2009 Inter-Research Science Center. This manuscript is available at http://www.int-res.com/ and may be cited as: Jacoby, C. A., Youngbluth, M. J., Frost, J. R., Flood, P. R., Uiblein, F., Båmstedt, U., Pagès, F., & Shale, D. (2009). Vertical distribution, behavior, chemical composition and metabolism of Stauroteuthis syrtensis (Octopoda:Cirrata) in the Northwest Atlantic. Aquatic Biology, 5(1), 13-22. doi:10.3354/ab00117. _- - Vol. 5: 13-22, 2009 AQUATIC BIOLOGY Printed March 2009 Published online Pebrusrv s. 2009 doi: 10.3354/ab00117 Aquat BioI 1 Vertical distribution, behavior, chemical composition and metabolism of Stauroteuthis svrtensis (Octopoda: Cirrata) in the northwest Atlantic 3 Charles A. Jacoby':", Marsh J. Younqbluth", Jessica R. Prost':", Per R. Flood , 4 Franz Uiblein , Ulf Bamstedt", Francese Paqes'':", David Shale? 'University of Florida, Gainesville, Florida 32653, USA '!IIMhor Branch Oceanoqraphlc InsliLution at Florida Atlantic University, 5600 Highway 1 North, Fort Pierce, Florida 34946, USA "Bathybtoloqica A/S, Gerhard Grans vei 58, 5081 Bergen, Norway "Institute of Marine Research, Nordnesgaten 33, PB 1870 Nordnes, 5817 Bergen, Norway sUmeiI Marine Sciences Centre, Norrbyn, 910 20 Hornetors, Sweden "Institut de Ciencies del Mar (CSIC), Passeig Marilim de Ia Barceloneta 37-49,08003 Barcelona, Spain 714 Victoria Avenue, Swanage, Dorset BH19 IAN, UK "Present address: Institute for Hydrohiology and Fisheries Science, University of Hamburg, Olbersweg 24, 22767 Hamburg, Germany ABSTRACT: The cirrate octopod Stauroteuthis syrtensis is a mesopelagic species commonly collected in the North Atlantic. -

Genus-Level Phylogeny of Cephalopods Using Molecular Markers: Current Status and Problematic Areas
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchOnline at James Cook University Genus-level phylogeny of cephalopods using molecular markers: current status and problematic areas Gustavo Sanchez1,2, Davin H.E. Setiamarga3,4, Surangkana Tuanapaya5, Kittichai Tongtherm5, Inger E. Winkelmann6, Hannah Schmidbaur7, Tetsuya Umino1, Caroline Albertin8, Louise Allcock9, Catalina Perales-Raya10, Ian Gleadall11, Jan M. Strugnell12, Oleg Simakov2,7 and Jaruwat Nabhitabhata13 1 Graduate School of Biosphere Science, Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan 2 Molecular Genetics Unit, Okinawa Institute of Science and Technology, Okinawa, Japan 3 Department of Applied Chemistry and Biochemistry, National Institute of Technology—Wakayama College, Gobo City, Wakayama, Japan 4 The University Museum, The University of Tokyo, Tokyo, Japan 5 Department of Biology, Prince of Songkla University, Songkhla, Thailand 6 Section for Evolutionary Genomics, Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark 7 Department of Molecular Evolution and Development, University of Vienna, Vienna, Austria 8 Department of Organismal Biology and Anatomy, University of Chicago, Chicago, IL, United States of America 9 Department of Zoology, Martin Ryan Marine Science Institute, National University of Ireland, Galway, Ireland 10 Centro Oceanográfico de Canarias, Instituto Español de Oceanografía, Santa Cruz de Tenerife, Spain 11 Graduate School of Agricultural Science, Tohoku University, Sendai, Tohoku, Japan 12 Marine Biology & Aquaculture, James Cook University, Townsville, Queensland, Australia 13 Excellence Centre for Biodiversity of Peninsular Thailand, Prince of Songkla University, Songkhla, Thailand ABSTRACT Comprising more than 800 extant species, the class Cephalopoda (octopuses, squid, Submitted 19 June 2017 cuttlefish, and nautiluses) is a fascinating group of marine conchiferan mollusks. -

2021 Malacologia Final Version
Redescription of the flapjack octopod, Opisthoteuthis bruuni (Cephalopoda: Opisthoteuthidae) from the southeastern Pacific Ocean and evolutionary relationships of cirrate octopods M. Cecilia Pardo-Gandarillas1*, Mariana Díaz-Santana-Iturrios2, Mark Fenwick3, Roger Villanueva4, Christian M. Ibáñez2 1.- Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Las Palmeras 3425, Ñuñoa, Santiago, Chile. 2.- Departamento de Ecología y Biodiversidad, Facultad de Ciencias de la Vida, Universidad Andres Bello, Avenida República 440, Santiago, Chile. 3.- National Institute of Water and Atmospheric Research, Ltd., Wellington, New Zealand. 4.- Institut de Ciències del Mar (CSIC), Passeig Maritim de la Barceloneta 37-49, 08003-Barcelona, Spain. Morphology and phylogeny of Opisthoteuthis bruuni *Corresponding author: [email protected] CONFLICT OF INTEREST STATEMENT: The authors declare that there is no conflict of interest. ABSTRACT Cirrate octopods are considered to resemble the ancestor of all octopuses. Cirrates inhabit the deep ocean and are characterized by the presence of fins, a cartilaginous inner shell and a single row of suckers alternating with pairs of cirri thus comprising uniserial suckers and biserial cirri. The objective of this contribution is to improve the taxonomy of Opisthoteuthis bruuni from the southeastern Pacific Ocean and suggest a new hypothesis of cirrate phylogeny. Given that the most complete molecular data set for cirrates available in public databases is comprised almost exclusively of 16S 1 rRNA gene sequences, we compared morphological and mitochondrial gene 16S rRNA data (generated in this study) from the genus Opisthoteuthis from the southeastern Pacific Ocean. Additionally, we sequenced Opisthoteuthis chathamensis, Opisthoteuthis mero and Luteuthis dentatus from the southwestern Pacific, as along with Cirroctopus mawsoni from Antarctic waters.