Sönke Johnsen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Biology Research Biodiversity in Concert: Common, Uncommon
This article was downloaded by: On: 11 February 2010 Access details: Access Details: Free Access Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37- 41 M ortim er Street, London W lT 3JH, UK Marine Biology Research Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713735885 Marine Biology Research former# Santa tNrfQMMo Biodiversity in concert: Common, uncommon, and new species Tom Fenchel; Franz Uiblein Online publication date: 09 December 2009 To cite this ArticleFenchel, Tom and Uiblein, Franz(2010) 'Biodiversity in concert: Common, uncommon, and new species', Marine Biology Research, 6: 1, 1 — 5 To link to this Article: DOI:10.1080/17451000903468856 URL: http://dx.doi.org/10.1080/17451000903468856 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. -

Visual Perception in Jumping Spiders (Araneae,Salticidae)
Visual Perception in Jumping Spiders (Araneae,Salticidae) A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Biology at the University of Canterbury by Yinnon Dolev University of Canterbury 2016 Table of Contents Abstract.............................................................................................................................................................................. i Acknowledgments .......................................................................................................................................................... iii Preface ............................................................................................................................................................................. vi Chapter 1: Introduction ................................................................................................................................................... 1 Chapter 2: Innate pattern recognition and categorisation in a jumping Spider ........................................................... 9 Abstract ....................................................................................................................................................................... 10 Introduction ................................................................................................................................................................ 11 Methods ..................................................................................................................................................................... -

A Protocol for Online Documentation of Spider Biodiversity Inventories Applied to a Mexican Tropical Wet Forest (Araneae, Araneomorphae)
Zootaxa 4722 (3): 241–269 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4722.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:6AC6E70B-6E6A-4D46-9C8A-2260B929E471 A protocol for online documentation of spider biodiversity inventories applied to a Mexican tropical wet forest (Araneae, Araneomorphae) FERNANDO ÁLVAREZ-PADILLA1, 2, M. ANTONIO GALÁN-SÁNCHEZ1 & F. JAVIER SALGUEIRO- SEPÚLVEDA1 1Laboratorio de Aracnología, Facultad de Ciencias, Departamento de Biología Comparada, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Colonia Copilco el Bajo. C. P. 04510. Del. Coyoacán, Ciudad de México, México. E-mail: [email protected] 2Corresponding author Abstract Spider community inventories have relatively well-established standardized collecting protocols. Such protocols set rules for the orderly acquisition of samples to estimate community parameters and to establish comparisons between areas. These methods have been tested worldwide, providing useful data for inventory planning and optimal sampling allocation efforts. The taxonomic counterpart of biodiversity inventories has received considerably less attention. Species lists and their relative abundances are the only link between the community parameters resulting from a biotic inventory and the biology of the species that live there. However, this connection is lost or speculative at best for species only partially identified (e. g., to genus but not to species). This link is particularly important for diverse tropical regions were many taxa are undescribed or little known such as spiders. One approach to this problem has been the development of biodiversity inventory websites that document the morphology of the species with digital images organized as standard views. -

The Jumping Spiders (Araneae: Salticidae) of the Virginia Peninsula1
Vol. 98, No. 5, November & December 1987 235 THE JUMPING SPIDERS (ARANEAE: SALTICIDAE) OF THE VIRGINIA PENINSULA 1 2 C.L. Stietenroth, N.V. Horner ABSTRACT: Thirty species representing 1 8 genera of Salticidae are recorded from the Virginia Peninsula. Habitat and natural history information for each species is presented. Some salticids on the peninsula occupy diverse habitats while other species appear to confine themselves to more restricted environments. The most abundant salticid was Hentzia palmarum. Metaphi- dippus galathea and Platycryptus undatus were most widely distributed species. Salticids reported in Virginia for the first time are Phidippus princeps, P. otiosus, Thiodina sylvana, Sitticus fasciger and Zygoballus sexpunctatus. A few studies concerning the spider fauna of Virginia have been published. The earliest record of occurrence was by John Banister between 1678 and 1692 (Ewan and Ewan, 1970). More recently, McCaffrey and Hornsburgh published three studies concerning spiders in apple orchards in central Virginia. Their assessment of spider populations in an unsprayed orchard was published in 1 1 977 followed ( 978) by laboratory feeding studies performed to evaluate potential effects of predaceous spiders on insect residents of apple orchards. Later (1980), a comparison was made between the spider populations in abandoned and commercial orchards; 68 species were identified. Dowd and Kok (1981), and McPherson el al. (1982) considered spider and other arthropod predation on the curculionid beetle, Rhynocyllus sp., in a in 1 soybean cropping system Virginia. Holsinger ( 982) reported on the spider cave-fauna in Burnsville Cove. The efficiency of limb-beating for capturing various spider families in apple orchards is discussed by McCaffrey and Parrella(1984). -

Zootaxa, New Lapsiine Jumping Spiders from Ecuador (Araneae
Zootaxa 1255: 17–28 (2006) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1255 Copyright © 2006 Magnolia Press ISSN 1175-5334 (online edition) New lapsiine jumping spiders from Ecuador (Araneae: Salticidae) WAYNE P. MADDISON Departments of Zoology and Botany and Centre for Biodiversity Research, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia, V6T 1Z4, Canada. Abstract Two new genera and three new species of salticid spider from eastern Ecuador are described, belonging to a group informally called "lapsiines". The new genus Galianora is based on Galianora sacha, new species, and also contains Galianora bryicola, new species. The new genus Thrandina includes the single new species Thrandina parocula. These genera share the ancestral salticid traits, rare among neotropical salticids, of a tarsal claw on the female palpus and a median apophysis on the male palp. Galianora is distinguished from other lapsiines by the round tegulum with peripheral embolus. The strikingly large posterior median eyes of Thrandina are unique among New World salticids. Key words: Araneae, Salticidae, Thrandina, Galianora, Lapsias, lapsiines, jumping spider, new species, Ecuador Introduction Phylogenetic studies of salticid spiders have revealed that most species fall in a single large clade, the Salticoida (Maddison & Hedin, 2003). The relatively few salticids outside of this clade therefore occupy a basal position in the family, and have been of considerable interest for studies of the early evolution of the family (Jackson & Pollard, 1996). While the Old World has about 25 genera of basal salticids of diverse body forms (Wanless, 1980, 1982, 1984; òabka & Kovac, 1996; Logunov, 2004), in the New World only Lyssomanes Hentz and Chinoscopus Simon have been recognized as basal (i.e., outside the Salticoida). -

SA Spider Checklist
REVIEW ZOOS' PRINT JOURNAL 22(2): 2551-2597 CHECKLIST OF SPIDERS (ARACHNIDA: ARANEAE) OF SOUTH ASIA INCLUDING THE 2006 UPDATE OF INDIAN SPIDER CHECKLIST Manju Siliwal 1 and Sanjay Molur 2,3 1,2 Wildlife Information & Liaison Development (WILD) Society, 3 Zoo Outreach Organisation (ZOO) 29-1, Bharathi Colony, Peelamedu, Coimbatore, Tamil Nadu 641004, India Email: 1 [email protected]; 3 [email protected] ABSTRACT Thesaurus, (Vol. 1) in 1734 (Smith, 2001). Most of the spiders After one year since publication of the Indian Checklist, this is described during the British period from South Asia were by an attempt to provide a comprehensive checklist of spiders of foreigners based on the specimens deposited in different South Asia with eight countries - Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan and Sri Lanka. The European Museums. Indian checklist is also updated for 2006. The South Asian While the Indian checklist (Siliwal et al., 2005) is more spider list is also compiled following The World Spider Catalog accurate, the South Asian spider checklist is not critically by Platnick and other peer-reviewed publications since the last scrutinized due to lack of complete literature, but it gives an update. In total, 2299 species of spiders in 67 families have overview of species found in various South Asian countries, been reported from South Asia. There are 39 species included in this regions checklist that are not listed in the World Catalog gives the endemism of species and forms a basis for careful of Spiders. Taxonomic verification is recommended for 51 species. and participatory work by arachnologists in the region. -

Vampire Squid Final
An Examination regarding the Phylogenetic Position of Vampyroteuthis infernalis Printing: Alex Dutton, Chase Klungle, Jake Nymeyer This poster is 48” wide by 36” high. It’s designed to be printed on a INTRODUCTION METHODS RESULTS large Vampire squid: • Research included 43 in-class taxa with one additional in-class outgroup. • Vampyroteuthis infernalis, “the living fossil” is found nested in-between the Vampyroteuthis infernalis, or the Vampire Squid, is a cephalopod found deep in the • Cross examination of two genes was implemented (H3 and Ribosomal 28s genes). suborder Cirrata (Octopuses) and the Order Oegopsida (squid) while exhibiting characteristics of both. ocean. It has 8 arms connected by a webbing or “cape,” and is typically black in color • Individual gene processing was accomplished utilizing Phylogeny.fr wherein: with red eyes. These attributes led to it being called a vampire (not because it drinks alignment of data was processed by MUSCLE, curation by Gblocks, Phylogeny analysis • This data shows V. infernalis as being contained within the monophyletic Customizing the Content: blood). This species exhibits traits that appear in both octopus and squid families by PhyML + aLRT, and initial tree rendering by TreeDyn. supergroup Octopodiformes. which results in a one-of-a-kind organism. However, the phylogenetic position of V. • SequenceMatrix was employed to combine the aligned gene sequences. • We can also note the increased evolutionary distance between V. infernalis and infernalis has yet to be truly defined. Some researchers believe that it aligns better squids as opposed to their closeness in previous research. The placeholders in this with squids while others side with its closeness to octopuses. -
Highlights Report with Live Links
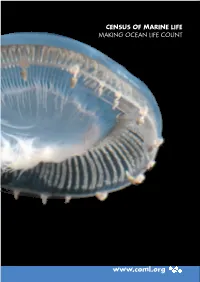
CENSUS OF MARINE LIFE MAKING OCEAN LIFE COUNT www.coml.org Census of Marine Life Projects Information System: OBIS Nearshore: NaGISA Coral Reefs: CReefs Regional Ecosystems: GoMA Continental Shelves: POST Continental Margins: COMARGE Abyssal Plains: CeDAMar Mid-Ocean Ridges: MAR-ECO Seamounts: CenSeam Vents and Seeps: ChEss Arctic Ocean: ArcOD Antarctic Ocean: CAML Top Predators: TOPP Zooplankton: CMarZ Microbes: ICoMM Oceans Past: HMAP Oceans Future: FMAP National and Regional Implementation Committees Australia Canada Caribbean China Europe Japan Indian Ocean Indonesia South Korea South America Sub-Saharan Africa United States Like an underwater spaceship, a jellyfish,Aequorea macrodactyla, travels through the warm, clear waters of the Celebes Sea in the Western Pacific Ocean. The jellyfish was but one of thousands of specimens photographed during a three-week Census expedition to explore this highly diverse area. Photo: Larry Madin, Woods Hole Oceanographic Institution. A Truly Global Endeavor The Census of Marine Life is a global network of researchers in more than 80 nations engaged in a ten-year scientific initiative to assess and ex- plain the diversity, distribution, and abundance of marine life in the oceans. The world’s first com- prehensive Census of Marine Life—past, present, and future—will be released in 2010. The Census gratefully acknowledges the financial support of numerous governments and organiza- tions from around the world. Moreover, many of the highlights in this report were only realized through the generous collaborative spirit and un- precedented cooperation of Census researchers and their international colleagues. A complete list of Census sponsors, funding partners, collaborat- ing institutions, and participating individuals is available at www.coml.org. -

Full Text in Pdf Format
Vol. 5: 13–22, 2009 AQUATIC BIOLOGY Printed March 2009 doi: 10.3354/ab00117 Aquat Biol Published online February 3, 2009 OPEN ACCESS Vertical distribution, behavior, chemical composition and metabolism of Stauroteuthis syrtensis (Octopoda: Cirrata) in the northwest Atlantic Charles A. Jacoby1,*, Marsh J. Youngbluth2, Jessica R. Frost1, 8, Per R. Flood3, Franz Uiblein4, Ulf Båmstedt5, Francesc Pagès6,†, David Shale7 1University of Florida, Gainesville, Florida 32653, USA 2Harbor Branch Oceanographic Institution at Florida Atlantic University, 5600 Highway 1 North, Fort Pierce, Florida 34946, USA 3Bathybiologica A/S, Gerhard Grans vei 58, 5081 Bergen, Norway 4Institute of Marine Research, Nordnesgaten 33, PB 1870 Nordnes, 5817 Bergen, Norway 5Umeå Marine Sciences Centre, Norrbyn, 910 20 Hörnefors, Sweden 6Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta 37–49, 08003 Barcelona, Spain 714 Victoria Avenue, Swanage, Dorset BH19 1AN, UK 8Present address: Institute for Hydrobiology and Fisheries Science, University of Hamburg, Olbersweg 24, 22767 Hamburg, Germany ABSTRACT: The cirrate octopod Stauroteuthis syrtensis is a mesopelagic species commonly collected in the North Atlantic. Individuals were observed at depths >600 m and typically within 100 m of the bot- tom in three ~900 m deep canyons indenting the southern edge of Georges Bank. When first sighted, most octopods were floating passively with their webbed arms gathered into a small ball. When dis- turbed, they expanded their webs to form a ‘balloon’ shape, swam slowly by sculling their fins, pulsed their webs like medusae and, in some cases, streamlined their arms and webs and moved away smoothly by rapidly sculling their fins. The bodies of 9 octopods comprised 92 to 95% water, with tissue containing 9 to 22% carbon (C) and 2 to 4% nitrogen (N). -

Araneae (Spider) Photos
Araneae (Spider) Photos Araneae (Spiders) About Information on: Spider Photos of Links to WWW Spiders Spiders of North America Relationships Spider Groups Spider Resources -- An Identification Manual About Spiders As in the other arachnid orders, appendage specialization is very important in the evolution of spiders. In spiders the five pairs of appendages of the prosoma (one of the two main body sections) that follow the chelicerae are the pedipalps followed by four pairs of walking legs. The pedipalps are modified to serve as mating organs by mature male spiders. These modifications are often very complicated and differences in their structure are important characteristics used by araneologists in the classification of spiders. Pedipalps in female spiders are structurally much simpler and are used for sensing, manipulating food and sometimes in locomotion. It is relatively easy to tell mature or nearly mature males from female spiders (at least in most groups) by looking at the pedipalps -- in females they look like functional but small legs while in males the ends tend to be enlarged, often greatly so. In young spiders these differences are not evident. There are also appendages on the opisthosoma (the rear body section, the one with no walking legs) the best known being the spinnerets. In the first spiders there were four pairs of spinnerets. Living spiders may have four e.g., (liphistiomorph spiders) or three pairs (e.g., mygalomorph and ecribellate araneomorphs) or three paris of spinnerets and a silk spinning plate called a cribellum (the earliest and many extant araneomorph spiders). Spinnerets' history as appendages is suggested in part by their being projections away from the opisthosoma and the fact that they may retain muscles for movement Much of the success of spiders traces directly to their extensive use of silk and poison. -

Riparian Spider Communities As Indicators of Stream Ecosystem Condition in the Río Piedras Watershed of Puerto Rico
Actual Biol Volumen 39 / Numero 107, 2017 Artículo científi co completo Riparian spider communities as indicators of stream ecosystem condition in the Río Piedras watershed of Puerto Rico Comunidades de arañas ribereñas como indicadores de la condición de los ecosistemas fluviales en la cuenca del Río Piedras de Puerto Rico Roberto Reyes-Maldonado1,3, José A. Sánchez-Ruiz2,4, Alonso Ramírez2,5 Sean P. Kelly*1,6 Abstract Human degradation of stream ecosystems has led to the creation of a number of methods to assess the severity of such anthropogenic impacts. Biomonitoring protocols that utilize aquatic organisms, in particular macroinvertebrates, are used worldwide as a way to evaluate stream ecosystems. Despite the various benefits these methods provide, they only take into account the stream channel, ignoring altogether the condition of the riparian zone. Other methods look at physical characteristics of both the riparian area and the stream, but ignore biota. Riparian consumers such as spiders have been proposed as potential bioindicators because they could provide a more holistic alternative for assessing stream impair- ment. Our aim was to determine whether changes in riparian spider communities could be used as indicators to separate sites with different levels of impact along an urban gradient. We conducted correlation analyses of riparian spider commu- nity metrics (abundance and species richness) and the percent of vegetation cover in subwatersheds with varying levels of urbanization, along with three other popular stream monitoring protocols. We found a clear difference in spider com- munity composition among subwatersheds, with an overall trend for lower richness and abundances in more impacted sites. -

(Arachnida: Scorpiones, Amblypygi and Araneae) Of
LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 PRELIMINARYLJL©2004 LJL©2004 LJL©2004 BIODIVERSITY LJL©2004 LJL©2004 LJL©2004ASSESSMENT LJL©2004 LJL©2004 AND LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004NOTES LJL©2004 ON LJL©2004THE BIOLOGY LJL©2004 LJL©2004 OF LJL©2004 THE ARACHNIDSLJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004