(Cephalopoda: Cirrata) Using a Minimally Invasive Approach Alexander Ziegler* and Christina Sagorny
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Biology Research Biodiversity in Concert: Common, Uncommon
This article was downloaded by: On: 11 February 2010 Access details: Access Details: Free Access Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37- 41 M ortim er Street, London W lT 3JH, UK Marine Biology Research Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713735885 Marine Biology Research former# Santa tNrfQMMo Biodiversity in concert: Common, uncommon, and new species Tom Fenchel; Franz Uiblein Online publication date: 09 December 2009 To cite this ArticleFenchel, Tom and Uiblein, Franz(2010) 'Biodiversity in concert: Common, uncommon, and new species', Marine Biology Research, 6: 1, 1 — 5 To link to this Article: DOI:10.1080/17451000903468856 URL: http://dx.doi.org/10.1080/17451000903468856 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. -

Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna, and Bonito in California Waters
UC San Diego Fish Bulletin Title Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters Permalink https://escholarship.org/uc/item/7t5868rd Authors Pinkas, Leo Oliphant, Malcolm S Iverson, Ingrid L.K. Publication Date 1970-06-01 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 152 Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters By Leo Pinkas , Malcolm S. Oliphant, and Ingrid L. K. Iverson 1971 1 2 ABSTRACT The authors investigated food habits of albacore, Thunnus alalunga, bluefin tuna, Thunnus thynnus, and bonito, Sarda chiliensis, in the eastern North Pacific Ocean during 1968 and 1969. While most stomach samples came from fish caught commercially off southern California and Baja California, some came from fish taken in central Califor- nia, Oregon, and Washington waters. Standard procedures included enumeration of food items, volumetric analysis, and measure of frequency of occur- rence. The authors identified the majority of forage organisms to the specific level through usual taxonomic methods for whole animals. Identification of partially digested animals was accomplished through the use of otoliths for fish, beaks for cephalopods, and the exoskeleton for invertebrates. A pictorial guide to beaks of certain eastern Pacific cephalopods was prepared and proved helpful in identifying stomach contents. This guide is presented in this publication. The study indicates the prominent forage for bluefin tuna, bonito, and albacore in California waters is the northern anchovy, Engraulis mordax. 3 ACKNOWLEDGMENTS The Food Habits Study of Organisms of the California Current System, (Project 6–7-R), was an investigation estab- lished under contract between the U.S. -

Octopoda: Opisthoteuthidae: Grimpoteuthis Sp.)
Marine Biology (2020) 167:82 https://doi.org/10.1007/s00227-020-03701-1 SHORT NOTE First in situ observation of Cephalopoda at hadal depths (Octopoda: Opisthoteuthidae: Grimpoteuthis sp.) Alan J. Jamieson1 · Michael Vecchione2 Received: 6 March 2020 / Accepted: 7 May 2020 / Published online: 26 May 2020 © The Author(s) 2020 Abstract The Cephalopoda are not typically considered characteristic of the benthic fauna at hadal depths (depths exceeding 6000 m), yet occasional open-net trawl samples have implied that they might be present to ~ 8000 m deep. Previous in situ photographic evidence has placed the deepest cephalopod at 5145 m. The discrepancies between the two have meant that the maximum depth for cephalopods has gone unresolved. In this study we report on unequivocal sightings, by HD video lander, of a cephalopod at hadal depths. The demersal cirrate octopod Grimpoteuthis sp. was observed at both 5760 and 6957 m in the Indian Ocean. These observations extend the known maximum depth range for cephalopods by 1812 m and increase the potential benthic habitat available to cephalopods from 75 to 99% of the global seafoor. Introduction which are known to attach their eggs to the seafoor, was found in the intestine of the snailfish Pseudoliparis The total bathymetric range of marine organisms is often (Careproctus) amblystomopsis from the same trench at difficult to resolve accurately because sampling effort 7210–7230 m (Birstein and Vinogradov 1955) which also becomes less frequent with increasing depth. One impor- indicated a hadal distribution (Akimushkin 1963). Finally, tant group with ambiguous records of maximum depth is the in 1975 a specimen of Grimpoteuthis sp. -

Xoimi AMERICAN COXCIIOLOGY
S31ITnS0NIAN MISCEllANEOUS COLLECTIOXS. BIBLIOGIIAPHY XOimi AMERICAN COXCIIOLOGY TREVIOUS TO THE YEAR 18G0. PREPARED FOR THE SMITHSONIAN INSTITUTION BY . W. G. BINNEY. PART II. FOKEIGN AUTHORS. WASHINGTON: SMITHSONIAN INSTITUTION. JUNE, 1864. : ADYERTISEMENT, The first part of the Bibliography of American Conchology, prepared for the Smithsonian Institution by Mr. Binuey, was published in March, 1863, and embraced the references to de- scriptions of shells by American authors. The second part of the same work is herewith presented to the public, and relates to species of North American shells referred to by European authors. In foreign works binomial authors alone have been quoted, and no species mentioned which is not referred to North America or some specified locality of it. The third part (in an advanced stage of preparation) will in- clude the General Index of Authors, the Index of Generic and Specific names, and a History of American Conchology, together with any additional references belonging to Part I and II, that may be met with. JOSEPH HENRY, Secretary S. I. Washington, June, 1864. (" ) PHILADELPHIA COLLINS, PRINTER. CO]^TENTS. Advertisement ii 4 PART II.—FOREIGN AUTHORS. Titles of Works and Articles published by Foreign Authors . 1 Appendix II to Part I, Section A 271 Appendix III to Part I, Section C 281 287 Appendix IV .......... • Index of Authors in Part II 295 Errata ' 306 (iii ) PART II. FOEEIGN AUTHORS. ( V ) BIBLIOGRxVPHY NOETH AMERICAN CONCHOLOGY. PART II. Pllipps.—A Voyage towards the North Pole, &c. : by CON- STANTiNE John Phipps. Loudou, ITTJc. Pa. BIBLIOGRAPHY OF [part II. FaliricillS.—Fauna Grcenlandica—systematice sistens ani- malia GrcEulandite occidentalis liactenus iudagata, &c., secun dum proprias observatioues Othonis Fabricii. -

The Lower Bathyal and Abyssal Seafloor Fauna of Eastern Australia T
O’Hara et al. Marine Biodiversity Records (2020) 13:11 https://doi.org/10.1186/s41200-020-00194-1 RESEARCH Open Access The lower bathyal and abyssal seafloor fauna of eastern Australia T. D. O’Hara1* , A. Williams2, S. T. Ahyong3, P. Alderslade2, T. Alvestad4, D. Bray1, I. Burghardt3, N. Budaeva4, F. Criscione3, A. L. Crowther5, M. Ekins6, M. Eléaume7, C. A. Farrelly1, J. K. Finn1, M. N. Georgieva8, A. Graham9, M. Gomon1, K. Gowlett-Holmes2, L. M. Gunton3, A. Hallan3, A. M. Hosie10, P. Hutchings3,11, H. Kise12, F. Köhler3, J. A. Konsgrud4, E. Kupriyanova3,11,C.C.Lu1, M. Mackenzie1, C. Mah13, H. MacIntosh1, K. L. Merrin1, A. Miskelly3, M. L. Mitchell1, K. Moore14, A. Murray3,P.M.O’Loughlin1, H. Paxton3,11, J. J. Pogonoski9, D. Staples1, J. E. Watson1, R. S. Wilson1, J. Zhang3,15 and N. J. Bax2,16 Abstract Background: Our knowledge of the benthic fauna at lower bathyal to abyssal (LBA, > 2000 m) depths off Eastern Australia was very limited with only a few samples having been collected from these habitats over the last 150 years. In May–June 2017, the IN2017_V03 expedition of the RV Investigator sampled LBA benthic communities along the lower slope and abyss of Australia’s eastern margin from off mid-Tasmania (42°S) to the Coral Sea (23°S), with particular emphasis on describing and analysing patterns of biodiversity that occur within a newly declared network of offshore marine parks. Methods: The study design was to deploy a 4 m (metal) beam trawl and Brenke sled to collect samples on soft sediment substrata at the target seafloor depths of 2500 and 4000 m at every 1.5 degrees of latitude along the western boundary of the Tasman Sea from 42° to 23°S, traversing seven Australian Marine Parks. -

First in Situ Observation of Cephalopoda at Hadal Depths (Octopoda: Opisthoteuthidae: Grimpoteuthis Sp.)
Marine Biology (2020) 167:82 https://doi.org/10.1007/s00227-020-03701-1 SHORT NOTE First in situ observation of Cephalopoda at hadal depths (Octopoda: Opisthoteuthidae: Grimpoteuthis sp.) Alan J. Jamieson1 · Michael Vecchione2 Received: 6 March 2020 / Accepted: 7 May 2020 / Published online: 26 May 2020 © The Author(s) 2020 Abstract The Cephalopoda are not typically considered characteristic of the benthic fauna at hadal depths (depths exceeding 6000 m), yet occasional open-net trawl samples have implied that they might be present to ~ 8000 m deep. Previous in situ photographic evidence has placed the deepest cephalopod at 5145 m. The discrepancies between the two have meant that the maximum depth for cephalopods has gone unresolved. In this study we report on unequivocal sightings, by HD video lander, of a cephalopod at hadal depths. The demersal cirrate octopod Grimpoteuthis sp. was observed at both 5760 and 6957 m in the Indian Ocean. These observations extend the known maximum depth range for cephalopods by 1812 m and increase the potential benthic habitat available to cephalopods from 75 to 99% of the global seafoor. Introduction which are known to attach their eggs to the seafoor, was found in the intestine of the snailfish Pseudoliparis The total bathymetric range of marine organisms is often (Careproctus) amblystomopsis from the same trench at difficult to resolve accurately because sampling effort 7210–7230 m (Birstein and Vinogradov 1955) which also becomes less frequent with increasing depth. One impor- indicated a hadal distribution (Akimushkin 1963). Finally, tant group with ambiguous records of maximum depth is the in 1975 a specimen of Grimpoteuthis sp. -

Arctic Cephalopod Distributions and Their Associated Predatorspor 146 209..227 Kathleen Gardiner & Terry A
Arctic cephalopod distributions and their associated predatorspor_146 209..227 Kathleen Gardiner & Terry A. Dick Biological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada Keywords Abstract Arctic Ocean; Canada; cephalopods; distributions; oceanography; predators. Cephalopods are key species of the eastern Arctic marine food web, both as prey and predator. Their presence in the diets of Arctic fish, birds and mammals Correspondence illustrates their trophic importance. There has been considerable research on Terry A. Dick, Biological Sciences, University cephalopods (primarily Gonatus fabricii) from the north Atlantic and the west of Manitoba, Winnipeg, Manitoba R3T 2N2, side of Greenland, where they are considered a potential fishery and are taken Canada. E-mail: [email protected] as a by-catch. By contrast, data on the biogeography of Arctic cephalopods are doi:10.1111/j.1751-8369.2010.00146.x still incomplete. This study integrates most known locations of Arctic cepha- lopods in an attempt to locate potential areas of interest for cephalopods, and the predators that feed on them. International and national databases, museum collections, government reports, published articles and personal communica- tions were used to develop distribution maps. Species common to the Canadian Arctic include: G. fabricii, Rossia moelleri, R. palpebrosa and Bathypolypus arcticus. Cirroteuthis muelleri is abundant in the waters off Alaska, Davis Strait and Baffin Bay. Although distribution data are still incomplete, groupings of cephalopods were found in some areas that may be correlated with oceanographic variables. Understanding species distributions and their interactions within the ecosys- tem is important to the study of a warming Arctic Ocean and the selection of marine protected areas. -

Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries
Advances in Cephalopod Science:Biology, Ecology, Cultivation and Fisheries,Vol 67 (2014) Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book Advances in Marine Biology, Vol. 67 published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: Paul G.K. Rodhouse, Graham J. Pierce, Owen C. Nichols, Warwick H.H. Sauer, Alexander I. Arkhipkin, Vladimir V. Laptikhovsky, Marek R. Lipiński, Jorge E. Ramos, Michaël Gras, Hideaki Kidokoro, Kazuhiro Sadayasu, João Pereira, Evgenia Lefkaditou, Cristina Pita, Maria Gasalla, Manuel Haimovici, Mitsuo Sakai and Nicola Downey. Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries. In Erica A.G. Vidal, editor: Advances in Marine Biology, Vol. 67, Oxford: United Kingdom, 2014, pp. 99-233. ISBN: 978-0-12-800287-2 © Copyright 2014 Elsevier Ltd. Academic Press Advances in CephalopodAuthor's Science:Biology, personal Ecology, copy Cultivation and Fisheries,Vol 67 (2014) CHAPTER TWO Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries Paul G.K. -

Opisthoteuthis Californiana Berry!
New Records and Observations on the Flapjack Devilfish, Opisthoteuthis californiana Berry! WALTER T. PEREYRA 2 IN JUNE 1961 the U. S. Bureau of Commercial respect to the development of secondary sexual Fisheries joined with the Atomic Energy Com characteristics ( Berry, 1954 and 1955 ) . The mission to undertake an investigation of the four specimens reported by Berry (two mature deep-water ocean fauna in the area contiguous females, one mature male, and a juvenile fe to the Columbia River mouth at depths from male ) represent the only previous records of 50 to 1050 fathoms (91-1920 m). The main this species known to the author. objectives of this project are to describe the Throughout the world, six other species of benthic fish and invertebrate communities in this genus have been described . Two species habiting the study area, and to define their are known from the Atlantic (the genus type bathymetric distribution on a temporal basis. O. agassizii Verrill, 1883 and an apparent pe Associated with the faunal investigation is a lagic species, O. m edusoides Thiele, 1915), one monitoring of the various demersal forms to from the Indian ocean off the west coast of evaluate the biological transport of radionucle Sumatra ( 0 . extensa Thiele, 1915 ), two from ids which may have their origin in Columbia Australian waters ( 0. persephone Berry, 1918 River waters. and O. pluto Berry, 1918), and one which has Thirty-one specimens of the flapjack devil been taken repeatedly in Japanese waters ( 0 . fish, Opisthoteuthis californiana, have been depressa Ijima and Ikeda, 1895). captured since initiation of the field program Despite wide interest in the taxonomy and in 1961. -

Vampire Squid Final
An Examination regarding the Phylogenetic Position of Vampyroteuthis infernalis Printing: Alex Dutton, Chase Klungle, Jake Nymeyer This poster is 48” wide by 36” high. It’s designed to be printed on a INTRODUCTION METHODS RESULTS large Vampire squid: • Research included 43 in-class taxa with one additional in-class outgroup. • Vampyroteuthis infernalis, “the living fossil” is found nested in-between the Vampyroteuthis infernalis, or the Vampire Squid, is a cephalopod found deep in the • Cross examination of two genes was implemented (H3 and Ribosomal 28s genes). suborder Cirrata (Octopuses) and the Order Oegopsida (squid) while exhibiting characteristics of both. ocean. It has 8 arms connected by a webbing or “cape,” and is typically black in color • Individual gene processing was accomplished utilizing Phylogeny.fr wherein: with red eyes. These attributes led to it being called a vampire (not because it drinks alignment of data was processed by MUSCLE, curation by Gblocks, Phylogeny analysis • This data shows V. infernalis as being contained within the monophyletic Customizing the Content: blood). This species exhibits traits that appear in both octopus and squid families by PhyML + aLRT, and initial tree rendering by TreeDyn. supergroup Octopodiformes. which results in a one-of-a-kind organism. However, the phylogenetic position of V. • SequenceMatrix was employed to combine the aligned gene sequences. • We can also note the increased evolutionary distance between V. infernalis and infernalis has yet to be truly defined. Some researchers believe that it aligns better squids as opposed to their closeness in previous research. The placeholders in this with squids while others side with its closeness to octopuses. -
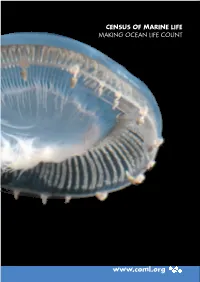
Highlights Report with Live Links
CENSUS OF MARINE LIFE MAKING OCEAN LIFE COUNT www.coml.org Census of Marine Life Projects Information System: OBIS Nearshore: NaGISA Coral Reefs: CReefs Regional Ecosystems: GoMA Continental Shelves: POST Continental Margins: COMARGE Abyssal Plains: CeDAMar Mid-Ocean Ridges: MAR-ECO Seamounts: CenSeam Vents and Seeps: ChEss Arctic Ocean: ArcOD Antarctic Ocean: CAML Top Predators: TOPP Zooplankton: CMarZ Microbes: ICoMM Oceans Past: HMAP Oceans Future: FMAP National and Regional Implementation Committees Australia Canada Caribbean China Europe Japan Indian Ocean Indonesia South Korea South America Sub-Saharan Africa United States Like an underwater spaceship, a jellyfish,Aequorea macrodactyla, travels through the warm, clear waters of the Celebes Sea in the Western Pacific Ocean. The jellyfish was but one of thousands of specimens photographed during a three-week Census expedition to explore this highly diverse area. Photo: Larry Madin, Woods Hole Oceanographic Institution. A Truly Global Endeavor The Census of Marine Life is a global network of researchers in more than 80 nations engaged in a ten-year scientific initiative to assess and ex- plain the diversity, distribution, and abundance of marine life in the oceans. The world’s first com- prehensive Census of Marine Life—past, present, and future—will be released in 2010. The Census gratefully acknowledges the financial support of numerous governments and organiza- tions from around the world. Moreover, many of the highlights in this report were only realized through the generous collaborative spirit and un- precedented cooperation of Census researchers and their international colleagues. A complete list of Census sponsors, funding partners, collaborat- ing institutions, and participating individuals is available at www.coml.org. -

Structure and Function of the Digestive System in Molluscs
Cell and Tissue Research (2019) 377:475–503 https://doi.org/10.1007/s00441-019-03085-9 REVIEW Structure and function of the digestive system in molluscs Alexandre Lobo-da-Cunha1,2 Received: 21 February 2019 /Accepted: 26 July 2019 /Published online: 2 September 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The phylum Mollusca is one of the largest and more diversified among metazoan phyla, comprising many thousand species living in ocean, freshwater and terrestrial ecosystems. Mollusc-feeding biology is highly diverse, including omnivorous grazers, herbivores, carnivorous scavengers and predators, and even some parasitic species. Consequently, their digestive system presents many adaptive variations. The digestive tract starting in the mouth consists of the buccal cavity, oesophagus, stomach and intestine ending in the anus. Several types of glands are associated, namely, oral and salivary glands, oesophageal glands, digestive gland and, in some cases, anal glands. The digestive gland is the largest and more important for digestion and nutrient absorption. The digestive system of each of the eight extant molluscan classes is reviewed, highlighting the most recent data available on histological, ultrastructural and functional aspects of tissues and cells involved in nutrient absorption, intracellular and extracellular digestion, with emphasis on glandular tissues. Keywords Digestive tract . Digestive gland . Salivary glands . Mollusca . Ultrastructure Introduction and visceral mass. The visceral mass is dorsally covered by the mantle tissues that frequently extend outwards to create a The phylum Mollusca is considered the second largest among flap around the body forming a space in between known as metazoans, surpassed only by the arthropods in a number of pallial or mantle cavity.