61–64. X-Rays (Title B)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuclear Medicine for Medical Students and Junior Doctors
NUCLEAR MEDICINE FOR MEDICAL STUDENTS AND JUNIOR DOCTORS Dr JOHN W FRANK M.Sc, FRCP, FRCR, FBIR PAST PRESIDENT, BRITISH NUCLEAR MEDICINE SOCIETY DEPARTMENT OF NUCLEAR MEDICINE, 1ST MEDICAL FACULTY, CHARLES UNIVERSITY, PRAGUE 2009 [1] ACKNOWLEDGEMENTS I would very much like to thank Prof Martin Šámal, Head of Department, for proposing this project, and the following colleagues for generously providing images and illustrations. Dr Sally Barrington, Dept of Nuclear Medicine, St Thomas’s Hospital, London Professor Otakar Bělohlávek, PET Centre, Na Homolce Hospital, Prague Dr Gary Cook, Dept of Nuclear Medicine, Royal Marsden Hospital, London Professor Greg Daniel, formerly at Dept of Veterinary Medicine, University of Tennessee, currently at Virginia Polytechnic Institute and State University (Virginia Tech), Past President, American College of Veterinary Radiology Dr Andrew Hilson, Dept of Nuclear Medicine, Royal Free Hospital, London, Past President, British Nuclear Medicine Society Dr Iva Kantorová, PET Centre, Na Homolce Hospital, Prague Dr Paul Kemp, Dept of Nuclear Medicine, Southampton University Hospital Dr Jozef Kubinyi, Institute of Nuclear Medicine, 1st Medical Faculty, Charles University Dr Tom Nunan, Dept of Nuclear Medicine, St Thomas’s Hospital, London Dr Kathelijne Peremans, Dept of Veterinary Medicine, University of Ghent Dr Teresa Szyszko, Dept of Nuclear Medicine, St Thomas’s Hospital, London Ms Wendy Wallis, Dept of Nuclear Medicine, Charing Cross Hospital, London Copyright notice The complete text and illustrations are copyright to the author, and this will be strictly enforced. Students, both undergraduate and postgraduate, may print one copy only for personal use. Any quotations from the text must be fully acknowledged. It is forbidden to incorporate any of the illustrations or diagrams into any other work, whether printed, electronic or for oral presentation. -

Nuclear Pharmacy Quick Sample
12614-01_CH01-rev3.qxd 10/25/11 10:56 AM Page 1 CHAPTER 1 Radioisotopes Distribution for Not 1 12614-01_CH01-rev3.qxd 10/25/1110:56AMPage2 2 N TABLE 1-1 Radiopharmaceuticals Used in Nuclear Medicine UCLEAR Chemical Form and Typical Dosage P Distribution a b HARMACY Radionuclide Dosage Form Use (Adult ) Route Carbon C 11 Carbon monoxide Cardiac: Blood volume measurement 60–100 mCi Inhalation Carbon C 11 Flumazenil injection Brain: Benzodiazepine receptor imaging 20–30 mCi IV Q UICK Carbon C 11 Methionine injection Neoplastic disease evaluation in brain 10–20 mCi IV R Carbon C 11 forRaclopride injection Brain: Dopamine D2 receptor imaging 10–15 mCi IV EFERENCE Carbon C 11 Sodium acetate injection Cardiac: Marker of oxidative metabolism 12–40 mCi IV Carbon C 14 Urea Diagnosis of Helicobacter pylori infection 1 µCi PO Chromium Cr 51 Sodium chromate injection Labeling red blood cells (RBCs) for mea- 10–80 µCi IV suring RBC volume, survival, and splenic sequestration Cobalt Co 57 Cyanocobalamin capsules Diagnosis of pernicious anemia and 0.5 µCi PO Not defects of intestinal absorption Fluorine F 18 Fludeoxyglucose injection Glucose utilization in brain, cardiac, and 10–15 mCi IV neoplastic disease Fluorine F 18 Fluorodopa injection Dopamine neuronal decarboxylase activity 4–6 mCi IV in brain Fluorine F 18 Sodium fluoride injection Bone imaging 10 mCi IV Gallium Ga 67 Gallium citrate injection Hodgkin’s disease, lymphoma 8–10 mCi IV Acute inflammatory lesions 5 mCi IV Indium In 111 Capromab pendetide Metastatic imaging in patients with biopsy- -

Internal and External Exposure Exposure Routes 2.1
Exposure Routes Internal and External Exposure Exposure Routes 2.1 External exposure Internal exposure Body surface From outer space contamination and the sun Inhalation Suspended matters Food and drink consumption From a radiation Lungs generator Radio‐ pharmaceuticals Wound Buildings Ground Radiation coming from outside the body Radiation emitted within the body Radioactive The body is equally exposed to radiation in both cases. materials "Radiation exposure" refers to the situation where the body is in the presence of radiation. There are two types of radiation exposure, "internal exposure" and "external exposure." External exposure means to receive radiation that comes from radioactive materials existing on the ground, suspended in the air, or attached to clothes or the surface of the body (p.25 of Vol. 1, "External Exposure and Skin"). Conversely, internal exposure is caused (i) when a person has a meal and takes in radioactive materials in the food or drink (ingestion); (ii) when a person breathes in radioactive materials in the air (inhalation); (iii) when radioactive materials are absorbed through the skin (percutaneous absorption); (iv) when radioactive materials enter the body from a wound (wound contamination); and (v) when radiopharmaceuticals containing radioactive materials are administered for the purpose of medical treatment. Once radioactive materials enter the body, the body will continue to be exposed to radiation until the radioactive materials are excreted in the urine or feces (biological half-life) or as the radioactivity weakens over time (p.26 of Vol. 1, "Internal Exposure"). The difference between internal exposure and external exposure lies in whether the source that emits radiation is inside or outside the body. -

Uroradiology Tutorial for Medical Students Lesson 3: Cystography & Urethrography – Part 1
Uroradiology Tutorial For Medical Students Lesson 3: Cystography & Urethrography – Part 1 American Urological Association Introduction • Conventional radiography of the urinary tract includes several diagnostic studies: – Cystogram – Retrograde urethrogram – Voiding cystourethrogram • All of these studies answer questions that are essential to urologic patient management Voiding Cystourethrogram (VCUG) • The voiding cystourethrogram is a dynamic test used to define the anatomy and, in part, the function of the lower urinary tract. It is performed by placing a catheter through the urethra into the bladder, filling the bladder with contrast material and then taking x-rays while the patient voids. You can imagine how popular it is among children. Scout Film • Several films are taken when performing a VCUG. The first image is a KUB called the scout film. On this film one can evaluate the bones of the spine and pelvis (injury or congenital anomaly such as spina bifida) and the soft tissues (calcifications, foreign bodies, etc.). • Normal scout image • What gender? Scout Film • Patients with urologic problems (urine infection or incontinence) may have a spinal abnormality that results in abnormal innervation of the bladder. Such anomalies are commonly associated with anomalies of the vertebral column. Let’s look at some spines. • Here is a spine from a normal KUB or scout film. Notice that the posterior processes of all the vertebrae are intact. You can see the posterior process behind and below each vertebral body. • Here is another scout film. Notice that the posterior processes are absent below L-4. This patient has lower lumbar spina bifida. Read This Scout Film • The bones are normal • What about soft tissues (bowel, etc.)? • This child has significant constipation. -
Mean Glandular Dose from Routine Mammography
Naresuan University Journal 2006; 14(3): 19-26 19 Mean Glandular Dose from Routine Mammography Supawitoo Sookpenga,* and Potjana Kettedb a Department of Radiological Technology, Faculty of Allied Health Science, Naresuan University, Phitsanulok 65000, Thailand. b Department of Radiology, Buddhachinaraj Hospital, Phitsanulok 65000, Thailand. *Corresponding author. E-mail address: [email protected] (S. Sookpeng) Received 21 July 2006; accepted 6 December 2006 Abstract A mammography examination facilitates the early detection of breast cancer. However, the potential risk of radiation-induced carcinogenesis is also increased with such a procedure. The objective of this research was to measure the mean glandular dose (MGD) from craniocaudal (CC) and mediolateral oblique (MLO) views in each breast and the total dose per woman (for both breasts and two views) from the exposure factor in patients undergoing mammography on six mammography x-ray generators in the lower region of northern Thailand. The values of compressed breast thickness (CBT), as well as the MGD calculated from the exposure and tube voltage both mAs and target/filter combination, were collected from 2,060 films from 515 women ranging in age from 28 to 91 years. Significant differences were found between MGD from CC and MLO projections. The MGD per film was 1.42+0.80 mGy for the CC projection and 1.56+0.86 mGy for the MLO projection, (p<0.001). The MGD per CC and MLO film was significantly related to CBT (r = 0.610, p<0.01 and r = 0.596, p<0.01 respectively). The result indicated that 96.1 % of CC films and 94.2 % of MLO films had doses less than 3.0 mGy as recommended by the American College of Radiology recommendations. -

FDA-Approved Radiopharmaceuticals
Medication Management FDA-approved radiopharmaceuticals This is a current list of all FDA-approved radiopharmaceuticals. USP <825> requires the use of conventionally manufactured drug products (e.g., NDA, ANDA) for Immediate Use. Nuclear medicine practitioners that receive radiopharmaceuticals that originate from sources other than the manufacturers listed in these tables may be using unapproved copies. Radiopharmaceutical Manufacturer Trade names Approved indications in adults (Pediatric use as noted) 1 Carbon-11 choline Various - Indicated for PET imaging of patients with suspected prostate cancer recurrence based upon elevated blood prostate specific antigen (PSA) levels following initial therapy and non-informative bone scintigraphy, computerized tomography (CT) or magnetic resonance imaging (MRI) to help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation 2 Carbon-14 urea Halyard Health PYtest Detection of gastric urease as an aid in the diagnosis of H.pylori infection in the stomach 3 Copper-64 dotatate Curium Detectnet™ Indicated for use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adult patients 4 Fluorine-18 florbetaben Life Molecular Neuraceq™ Indicated for Positron Emission Tomography (PET) imaging of the brain to Imaging estimate β amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) or other causes of cognitive decline 5 Fluorine-18 -

Radionuclide Cystography 138
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice guidelines and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice guidelines and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice guideline and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review, requiring the approval of the Commission on Quality and Safety as well as the ACR Board of Chancellors, the ACR Council Steering Committee, and the ACR Council. The practice guidelines and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice guideline and technical standard by those entities not providing these services is not authorized. Revised 2010 (Res. 25)* ACR–SPR–SNM PRACTICE GUIDELINE FOR THE PERFORMANCE OF ADULT AND PEDIATRIC RADIONUCLIDE CYSTOGRAPHY PREAMBLE These guidelines are an educational tool designed to assist Therefore, it should be recognized that adherence to these practitioners in providing appropriate radiologic care for guidelines will not assure an accurate diagnosis or a patients. -
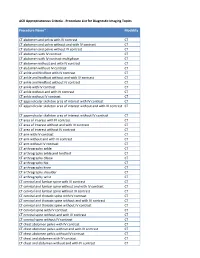
Standard Procedure List – AC Diagnostic Topics
ACR Appropriateness Criteria - Procedure List for Diagnostic Imaging Topics Procedure Name* Modality CT abdomen and pelvis with IV contrast CT CT abdomen and pelvis without and with IV contrast CT CT abdomen and pelvis without IV contrast CT CT abdomen with IV contrast CT CT abdomen with IV contrast multiphase CT CT abdomen without and with IV contrast CT CT abdomen without IV contrast CT CT ankle and hindfoot with IV contrast CT CT ankle and hindfoot without and with IV contrast CT CT ankle and hindfoot without IV contrast CT CT ankle with IV contrast CT CT ankle without and with IV contrast CT CT ankle without IV contrast CT CT appendicular skeleton area of interest with IV contrast CT CT appendicular skeleton area of interest without and with IV contrast CT CT appendicular skeleton area of interest without IV contrast CT CT area of interest with IV contrast CT CT area of interest without and with IV contrast CT CT area of interest without IV contrast CT CT arm with IV contrast CT CT arm without and with IV contrast CT CT arm without IV contrast CT CT arthrography ankle CT CT arthrography ankle and hindfoot CT CT arthrography elbow CT CT arthrography hip CT CT arthrography knee CT CT arthrography shoulder CT CT arthrography wrist CT CT cervical and lumbar spine with IV contrast CT CT cervical and lumbar spine without and with IV contrast CT CT cervical and lumbar spine without IV contrast CT CT cervical and thoracic spine with IV contrast CT CT cervical and thoracic spine without and with IV contrast CT CT cervical and thoracic spine -

Radionuclide Techniques for the Detection of Vesicoureteral Reflux
Review Article Radionuclide techniques for the detection of vesicoureteral reflux and their clinical significance Abstract We discuss and try to evaluate the detection of vesicoureteral reux (VUR) by radionuclide techniques and especially direct radionuclide cystography (DRC). Direct radionuclide cystography is applied for more than half a century mainly in children. Vesicoureteral reux has a complex pathology not yet completely under- Christodoulos Likartsis1 MD, BSc stood and is often related to urinary tract infection (UTI) and renal parenchyma scarring that can lead to 2 long-term renal function impairment. Since there is no consensus on the optimal imaging algorithm after Nikoleta Printza MD, PhD 1 the rst febrile urinary tract infection, many imaging strategies have been proposed for VUR detection in Athanasios Notopoulos MD, the last decade, including or not DRC. Views opposing or accepting its use are also presented. MDA, PhD Hell J Nucl Med 2020; 23(2): 180-187 Epub ahead of print: 27 July 2020 Published online: 24 August 2020 Introduction 1. Nuclear Medicine Department, Hippokration Hospital, Thessaloniki, Greece 2. 1st Pediatric Department, Aristotle University, Thessaloniki, bout 30% to 40% of children diagnosed with urinary tract infection (UTI), have Greece primary vesicoureteral reux (VUR) [1]. Children with VUR have 2.6 times higher Aprevalence of renal scarring compared to children without VUR [2]. For most of children with VUR, treatment involves continuous antibiotic prophylaxis (CAP). Mictura- ting cystourethrography (MCU) and direct radionuclide cystography (DRC) are mainly applied for the detection and follow-up of VUR respectively [3] although some authors are not agreeable as for their indications [4, 5]. -

Cystography You Will Change in to a Hospital Gown Welcome to VCU Health Diagnostic Imaging
Diagnostic Fluoroscopy Patient Information Cystography You will change in to a hospital gown Welcome to VCU Health Diagnostic Imaging. We are An abdominal x-ray will be obtained located in the Main Hospital 3rd floor, 1200 East Marshall The nurse or physician will insert a catheter into Street. Our hours of operation are Monday through the bladder Friday, 8 am to 5 pm. Advanced scheduling is required for all diagnostic fluoroscopy exams. Please review the You will be laying down on a fluoroscopy table following information about your test. Please call (804) while a contrast agent hooked up to the catheter 628-3580 to schedule your test or if you have any flows in to your bladder. The radiologist will take questions about your diagnostic fluoroscopy exam. images with the fluoroscopy x-ray machine What is it? Once your bladder is full, you will be asked to move in a few different positions Imaging of the urinary system. A bedpan or urinal will be given to you and you Why are you having this test? will be asked to urinate while the radiologist is You are having this study to establish presence or imaging with the fluoroscopy equipment absence, nature or extent of disease affecting the urinary A post void x-ray will be taken if you have not system. Imaging from cystography studies can reveal completely emptied your bladder abnormalities related to: After Care information Hematuria (blood in the urine) You may resume normal activity, eating and Recurring urinary tract infections (UTIs) drinking after exam unless otherwise specified by Leaking Urine your physician. -

Diagnostic Imaging; Nuclear Medicine; Radiation Risk Vs. Benefit
Diagnostic Imaging, Nuclear Medicine, Radiation Risks vs. Benefit & Radiation Protection (Chapter 15, 16, & 17) 1 Outline Background perspective Diagnostic radiology Interventional radiology Nuclear medicine Fetal & childhood dose 2 1 Internal Radiation People are exposed to radiation from radioactive material inside their bodies. Besides radon, the most important internal radioactive element is naturally occurring K-40, but uranium and thorium are also present as well as H-3 and C-14. The amount of radiation from potassium-40 does not vary much from one person to another. However, exposure from radon varies significantly from place to place depending on the amount of uranium in the soil. On average, in the United States radon contributes 55% of all radiation exposure from natural and man- made sources. Another 11% comes from the other radioactive materials inside the body. 2 Average Annual Effective Dose in US population (1982) mSv Natural Background Radon 2.0 other 1.0 Occupational 0.009 Medical diagnostic X-rays 0.39 nuclear medicine 0.14 __________________________________ Total (rounded) 3.6 mSv (360 mrem)/year From: Mettler et al., Ionizing Radiation From NCRP 1987 6 3 FIGURE 16.3 Color plot of the annual cosmic radiation doses (in microsievert) in North America. The variation with altitude is very clear, with the highest doses in the Rocky Mountains. Looking at Medical Exposures Patients Professionals Classes of Exposure in Medicine Diagnostic Interventional Radiology Nuclear Medicine Irradiation of Children and Pregnant Women 8 4 Diagnostic Radiology Surveys of dose from diagnostic exposures conducted with: NCRP 100 (1989) which contains a compilation of limited surveys, UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2000 Various other surveys and sources of dose info Diagnostic dose is certainly a potential for stochastic effects, deterministic effects very unlikely. -

Radiation Protection of the Public S15 Oral Presentations
Contents Session 15: Radiation protection of the public S15 Oral presentations S15-01 The main directions of the Russian efforts in radiation safety and health protection . 2180 Kiselev, Mikhail; Shandala, Nataliya S15-02 Spanish national campaign for the search and recovery of orphan radioactive sources . 2189 Carboneras, Pedro; Ortiz, Maria Teresa; Rueda, Carmen S15-03 Mitigation of exposure to radon by household water treatment . 2200 Turtiainen, Tuukka S15-04 Dose assessment for tritium releases during normal operation of NPP (ABSTRACT) . 2207 Duran, Juraj; Malátová, Irena S15-05 The legacy of uranium mines – Pluralist Expertise Group experiment on uranium mines in Limousin (France) (ABSTRACT) . 2208 Ringeard, Caroline; Catelinois, Olivier; Sene, Monique; Barbey, Pierre; Andres, Christian; Devin, Patrick; Vandenhove, Hildegarde; Servant-Perrier, Anne-Christine; Leuraud, Klervi; Zerbib, Jean-Claude Third European IRPA Congress 2010, Helsinki, Finland Contents Topic 15: Radiation protection of the public P15 Poster presentations P15-01 Radiation situation in Moscow and public doses due to man-made radiation exposure . 2210 Metlyaev, Evgeny; Filonova, Anna P15-02 Ionizing radiation exposure of the Belgian population in 2006 . 2216 Vanmarcke, Hans; Bosmans, Hilde; Eggermont, Gilbert P15-03 Sharing an environmental monitoring network: The AREVA Tricastin experience . 2226 Mercat, Catherine; Devin, Patrick; Garnier, François P15-04 137Cs activity concentrations in Polish meats – Current status and dose assessment for consumers . 2236 Rachubik, Jarosław P15-05 Public doses due to tritium emissions from Cernavoda NPP (ABSTRACT) . 2244 Bobric, Elena; Popescu, Ion; Simionov, Vasile P15-06 New Swedish regulations for clearance of materials, rooms, buildings and land . 2245 Efraimsson, Henrik P15-07 The approach to assessing doses to humans in the Posiva safety case .