Chapter – 1 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Details of Agreement's Executed in Form – III Sl. No Appl. No. Applicant
Details of Agreement’s Executed in Form – III Sl. Appl. Applicant Name & Contact For Name of the Project / Bioresources Agreement Patent No No. Details m Invention signed on No 1 100 M/s. Scitech Centre, III Invention relates to 1.Glycyrrhiza glabra 12.06.2007 7, Prabhat Nagar, composition and a process 2.Asparagus officinalis Jogeshwari (West), for preparation of a 3.Angelice officinalis Mumbai – 400 012, composition for 4.pimpinella anisum Maharashtra, manufacturing textured 5.Azdiracta indica 6.Acacia India. soluble container using catechu 7.Acorus calamus herbal texturing agent 8.Andrographis paniculata 9.Berberis asiatica 10.Bergenia cordifolia 11.Boerhaavia diffusa 12.Curcuma longa 13.Cuminum cyminum 14.Cinnamomum zilanicum 15.Coriandrum sativum 16.Centella asiatica 17. Clerodendrum paniculatum 18. Dioscorea bulbifera 19.Echinecea purpurea 20.Eclipta alba 21.Foeniculum vulgare 22.Gingiber officinale 23.Gymnema salvastre 24.hemidesmus indicus 25.Hydrastis urge or 26.Nardostachy jatamansi 27.Pueraria tuberose 28.Phyllanthus amarus 29.Picorrhiza kurroa 30.Pluchea lanceolata 31.Ricinus communis 32.Rauvolfia indica 33.Rubia cordifolia 34.Sida cordifolia 35.Saraca asoca 36.Saussurea lappa 37.Terminalia chebula 38.Tinospora cordifolia 39.Tylophora indica 40.Valeriana officinalis 41.Withiana somnifera 2 79 M/s. Vasundhara, III Water detoxication by Coconut Coir 21.06.2007 15, Saheed Nagar, using coconut coir Bhubaneswar – 751 007, Orissa, India. 3 80 M/s. Vasundhara, III Water detoxication by Bacha (Acorus calamus) 21.06.2007 15, Saheed Nagar, using bacha (Acorus rhizomes Bhubaneswar – 751 007, calamus) rhizomes extract Orissa, India. 4 81 M/s. Vasundhara, III Water detoxication by Jamun seed (Syzygium cumini 21.06.2007 15, Saheed Nagar, using Syzygium cumini (L.) Skeels) Bhubaneswar – 751 007, seed extract. -

Storage Plans for Value Added MFP Products – Van Dhan
Storage Plans for Value Added MFP Products – Van Dhan Storage Guidelines for MFP and Value Added Products - PMVDY After the processing of Minor Forest Produce (MFP) in Van Dhan Vikas Kendras (VDVKs), the need for storing the value added products becomes imperative as they must be preserved until needed for consumption. This document provides guidelines specifying how locally processed items should be preserved. Proper Storage Requirements: Any given storage system must be easy for maintenance and management. A good storage must be prevented from moisture and excessive air current. A good storage system enables free access in terms of regular check to access the state of the product. Any stored produce must be protected from pests, rodents and birds by allowing proper storage hygiene and maintenance. The storage facility must give ease of loading and offloading as the need arises. This is to create accessibility of the product. Storage Methods: Many people store their produce in the drying place itself which is not the right way of storage. Often the root or leaves are not dried properly which can cause harm if not stored properly. It is best to transfer the clean, dry produce to a cool, moisture-free place where rats and insects cannot follow. Warehouses are large houses or spaces that are commonly used as storage structures. They are especially constructed for the protection of the quantity and quality of processed agricultural products. They ways in which processed items can be stored are as follows: Bag Storage: This is a very popular form of storage. Transportation of the product is done in jute bags, the bags are easy to handle and the jute bag allows you to store different items in the same room. -

3.Eng-Non-Edible Kusum Oil Potential Foliage of Biodiesel Production and Its Productive
IMPACT: International Journal of Research in Engineering & Technology (IMPACT: IJRET) ISSN(P): 2347-4599; ISSN(E): 2321-8843 Vol. 4, Issue 9, Sep 2016, 25-36 © Impact Journals NON-EDIBLE KUSUM OIL: POTENTIAL FOLIAGE OF BIODIESEL PRODUCTION AND ITS PRODUCTIVE USE IN MARINE ENGINES SUDHANSU BHUSAN MOHAPATRA 1, PREMANANDA DAS 2, RAMESH CHANDRA MOHANTY 3 & DHANESWAR SWAIN 4 1Assistant Professor, Centurion University of Technology and Management, Jatni, Bhubaneswar, Odisha, India 2Founder of Research Foundation for Rural and Tribal Resource Development, Bhubaneswar, Odisha, India 3Professor, Centurion University, Odisha, India 4Research Scholar, Centurion University, Odisha India ABSTRACT Biodiesel from non-edible vegetable oils is of paramount significance in India due to insufficient edible oil production. The use of biodiesel has been widely accepted as an effective solution to reduce greenhouse emissions. The high potential of biodiesel in terms of PM, NO x, CO and CO 2 emission reduction may represent an additional motivation for its wide use. However the poor low temperature operability is imperative. According to these observations a different behaviour of the after treatment system, especially as far as control issues of the Diesel Particulate Filter are concerned is also expected. The use of biodiesel as alternative to fossil fuel for light duty CI engines to reduce greenhouse gas emissions was widely investigated. However, poor stability of biodiesel - diesel mixture limits the use of biodiesel to low volume concentrations. This paper presents the results concerning the use of a novel fuel additive package containing, pour-point depressant with the aim to increase the quality and amount of biodiesel in the diesel-biodiesel blends. -

A Art of Essential Oils
The Essence’s of Perfume Materials Glen O. Brechbill FRAGRANCE BOOKS INC. www.perfumerbook.com New Jersey - USA 2009 Fragrance Books Inc. @www.perfumerbook.com GLEN O. BRECHBILL “To my parents & brothers family whose faith in my work & abilities made this manuscript possible” II THE ESSENCES OF PERFUME MATERIALS © This book is a work of non-fiction. No part of the book may be used or reproduced in any manner whatsoever without written permission from the author except in the case of brief quotations embodied in critical articles and reviews. Please note the enclosed book is based on The Art of Fragrance Ingredients ©. Designed by Glen O. Brechbill Library of Congress Brechbill, Glen O. The Essence’s of Perfume Materials / Glen O. Brechbill P. cm. 477 pgs. 1. Fragrance Ingredients Non Fiction. 2. Written odor descriptions to facillitate the understanding of the olfactory language. 1. Essential Oils. 2. Aromas. 3. Chemicals. 4. Classification. 5. Source. 6. Art. 7. Thousand’s of fragrances. 8. Science. 9. Creativity. I. Title. Certificate Registry # 1 - 164126868 Copyright © 2009 by Glen O. Brechbill All Rights Reserved PRINTED IN THE UNITED STATES OF AMERICA 10 9 8 7 6 5 4 3 2 1 First Edition Fragrance Books Inc. @www.perfumerbook.com THE ESSENCE’S OF PERFUME MATERIALS III My book displays the very best of essential oils. It offers a rich palette of natural ingredients and essences. At its fullest it expresses a passion for the art of perfume. With one hundred seventy-seven listings it condenses a great deal of pertinent information in a single text. -

Experimental Investigation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi Method
Advances in Chemical Engineering and Science, 2017, 7, 464-476 http://www.scirp.org/journal/aces ISSN Online: 2160-0406 ISSN Print: 2160-0392 Experimental Investigation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi Method Rabiranjan Murmu1,2, Harekrushna Sutar1*, Sangram Patra1 1Chemical Engineering Department, Indira Gandhi Institute of Technology, Sarang, India. 2Chemical Engineering Department, Indian Institute of Technology, Madras, India How to cite this paper: Murmu, R., Sutar, H. and Patra, S. (2017) Experimental Investi- Abstract gation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi The paper focuses on biodiesel production from kusum oil using esterification Method. Advances in Chemical Engineering reaction followed by transesterification reaction in an in-house batch reactor and Science, 7, 464-476. setup. The effects of methanol to oil ratio (M/O), catalyst amount (H SO and https://doi.org/10.4236/aces.2017.74033 2 4 methodoxide) and reaction temperature on acid value and fatty acid methyl Received: September 19, 2017 esters (FAME) is studied. Product has been analysed using FTIR spectroscopy Accepted: October 28, 2017 technique for confirmation of ester group in biodiesel. Experimental data was Published: October 31, 2017 optimized by Taguchi analysis to conclude the optimum variable affecting the Copyright © 2017 by authors and response. In both processes M/O ratio has the significant effect for biodiesel Scientific Research Publishing Inc. production. The obtained biodiesel properties are close to commercial diesel This work is licensed under the Creative fuel and may be rated as an alternative to conventional diesel. The biodiesel Commons Attribution International License (CC BY 4.0). -

CB-NRM Technical Manual Vol
CB-NRM Technical Manual Vol. 3: Income Generating/Livelihood Development Prepared by The Project for Community-Based Sustainable Natural Resource Management in the Democratic Republic of Timor-Leste Project for Community-Based Sustainable Natural Resource Management in the Democratic Republic of Timor-Leste CB-NRM Technical Manual Vol. 3: Income Generating / Livelihood Development Table of Contents page Chapter 1 Introduction ............................................................................................ 1 1.1 Rationale for the Techniques................................................................................ 1 1.2 Objectives of the Techniques ............................................................................... 1 1.3 Objectives of the Manual ..................................................................................... 1 Chapter 2 Approaches to Effective Transferring of Techniques ......................... 2 2.1 Hands-on Training and Follow-up On-the Job Training (OJT) ........................... 2 2.2 Resource-Based ................................................................................................... 3 2.3 Participatory ......................................................................................................... 3 2.4 Women Centric .................................................................................................... 3 2.5 Continuous Coaching ........................................................................................... 3 2.6 Framework to Transfer -

WRA Species Report
Family: Sapindaceae Taxon: Schleichera oleosa Synonym: Schleichera trijuga Willd. Common Name: Ceylon oak lactree Macassar oiltree Malay lactree Questionaire : current 20090513 Assessor: HPWRA OrgData Designation: EVALUATE Status: Assessor Approved Data Entry Person: HPWRA OrgData WRA Score 1 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 n 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see n Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 n 405 Toxic to animals -
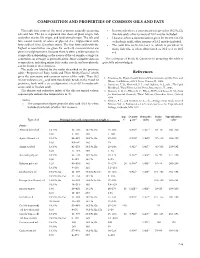
Composition and Properties of Common Oils and Fats References
COMPOSITION AND PROPERTIES OF COMMON OILS AND FaTS This table lists some of the most common naturally occurring • In some oils where a concentration is given for 18:2 9c,12c oils and fats . The list is separated into those of plant origin, fish (linoleic acid), other isomers of 18:2 may be included . and other marine life origin, and land animal origin . The oils and • Likewise, where a concentration is given for 18:3 9c,12c,15c fats consist mainly of esters of glycerol (i .e ., triglycerides) with (α-linolenic acid), other isomers of 18:3 may be included . fatty acids of 10 to 22 carbon atoms . The four fatty acids with the • The acid 20:5 6c,9c,12c,15c,17c, which is prevalent in highest concentration are given for each oil; concentrations are many fish oils, is often abbreviated as 20:5 ω-3 or 20:5 given in weight percent . Because there is often a wide variation in n-3 . composition depending on the source of the oil sample, a range (or sometimes an average) is generally given . More complete data on The assistance of Frank D . Gunstone in preparing this table is composition, including minor fatty acids, sterols, and tocopherols, gratefully acknowledged . can be found in the references . The acids are labeled by the codes described in the previous table, “Properties of Fatty Acids and Their Methyl Esters,” which References gives the systematic and common names of the acids . Thus 18:2 1 . Firestone, D ., Physical and Chemical Characteristics of Oils, Fats, and 9c,12c indicates a C18 acid with two double bonds in the 9 and 12 Waxes, 2nd Edition, AOCS Press, Urbana, IL, 2006 . -

Value Addition Processing of Mfps
Value Addition & Processing of Minor Forest Produce Value Chain for MFP – Stages The following commodity was studied extensively for the purpose of identifying the stages of value addition – Bael Pulp (dried) (Aegle marmelos) [ MSP – Rs. 27/kg ] Raw Material (Collection): The fruit should be picked individually, so that it does not fall on the ground to avoid cracking of the shell which may lead to spoilage during storage. Normally, the fruit should be harvested when yellowish-green and keep for 8 days while it loses its green tint. Then the stem readily separates from the fruit. A clear, gummy sap, resembling gum arabic, exudes from wounded branches and hangs down in long strands, becoming gradually solid. The fruits need to be harvested in January (2 to 3 months before full maturity) and ripened artificially in 18 to 24 days by treatment with 1,000 to 1,500 ppm ethrel (2-chloroethane phosphonic acid) and storage at 86º F (30º C). Care is needed in harvesting and handling to avoid causing cracks in the rind. Fruits need to be harvested by using long sticks attached with a hook and small bag around it. As a thumb rule, 1/3rd of the fruits should be left for consumption by birds and other animals for regeneration purpose. Cutting the branches or the whole tree for harvesting should be avoided. Ripen fruits can be harvested by shaking the branches. The hard shells of harvested fruits need to be breached by striking with a wooden or iron or stone hammer. The pulp should be removed by using a clean steel spoon or spatula and kept in clean clothes/ bamboo/ grass mats in the sun. -

Non-Wood Forest Products in Asia
Page 1 of 4 Non -Wood Forest Products in Asia RAPA PUBLICATION 1994/28 REGIONAL OFFICE FOR ASIA AND THE PACIFIC (RAPA) FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS BANGKOK 1994 EDITORS Patrick B. Durst Ward Ulrich M. Kashio The designations and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The opinions expressed in this publication are those of the authors alone and do not imply any opinion whatsoever on the part of FAO. COVER PHOTO CREDIT: Mr. K. J. Joseph PHOTO CREDITS: Mr. Mohammad Iqbal Sial Mr. A.L. Rao Mr. Urbito Oncleo Mr. Michael Jensen Mr. K. J. Joseph EDITED BY: Mr. Patrick B. Durst Mr. Ward Ulrich Mr. M. Kashio TYPE SETTING AND LAYOUT OF PUBLICATION: Helene Praneet Guna-Tilaka FOR COPIES WRITE TO: FAO Regional Office for Asia and the Pacific 39 Phra Atit Road Bangkok 10200 Page 2 of 4 Table of Contents Foreword Bangladesh Introduction Major NWFPs Collection and processing of NWFPs Economic and social benefits from NWFPs Promoting NWFPs References China Introduction Production and utilization of non-wood forest products Prospects for NWFP development in China India Introduction Status of various categories of non-wood forest products Export of non-wood forest products Organization for collection and processing Employment generation and -

Official (;Azette Go\'Ernment of Goa
l REG». GOA· 5 , Panaji, 2nd February, 1990 (Magha 13, 1911) SERIES I No. 44 OFFICIAL (;AZETTE GO\'ERNMENT OF GOA EX Tft f\ 0 ft D liN 1\ RY / GOVERNMENT OF GOA. DAMAN Where, in respect of any goods, the Cen tral Government is satisfied that .the duty AND DIU leviable lhereon under section 3 of the law Department Central E:xcisesand Salt Act, 1944 should 1 of 1944. be increa;,ed and that circumstances exist Legal Affairs Branch which render it necessary to ta.1re imme- d'2.te action, the' Central Government may, by notification in the Official Notification Gazette, direct an amendment of the LD/1/96·L.A.B. Schedule to be made so as to subsUtute for the ',ate of duty specified in the The Central Excise Tariff Act, 1985 (No. 5 of Schedule in respect of such goods,- 1\)86) which was passed by Parliament and assented to by the President of Iildia on 19th January, 1986 (a). in a case where the rate of duty as specified and published in the Gazette of India Extraordinary, in the Schedule as in force immediately before Part II, Section 1 dated 20·1·86 is hereby republished the issue of such notification is nil, a rate of duty for the general information. not exceeding fifty per cent. ad valorem expressed B. S. Subbanna, Under Secretary to the' Govern in any form or method; ment of Goa, Daman and Diu. (b) in any other case, rate of duty which shali Pam.ji, 18th March, 1986. not be more than twice the rate of duty specified in respect of such goods in the Schedule as in force immediately before the issue of the said notifica- The Central Excise Tariff Act, 1985 ti=: . -

Forest Utilization
FOREST UTILIZATION DIRECTORATE OF FORESTS GOVERNMENT OF WEST BENGAL Forest Utilization 1 This edition is published by Development Circle, Directorate of Forests, Government of West Bengal, 2016 Aranya Bhavan LA – 10A Block, Sector III Salt Lake City, Kolkata, West Bengal, 700098 Copyright © 2016 in text Copyright © 2016 in design and graphics All rights reserved. No part of this publication may be reproduced, stored in any retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the copyright holders. 2 Forest Utilization Forest Utilization PREFACE Forestry Utilization is the subject that deals with harvest, processing and disposal of forest produce. Although the focus till now has been on wood products, particularly timber, the economic and social importance of various non-wood products is now duly appreciated in the sphere of forest management. Under the JICA project on ‘Capacity Development for Forest Management and Training of Personnel’ being implemented by the Forest Department, Govt of West Bengal, this course material on Forest Utilization has been prepared for induction training of the Foresters and Forest Guards. The object of this training manual is to help the frontline forest personnel have a better perception about basic principles and procedures involved in forest utilization. The subjects covered in these materials broadly conform to the syllabus laid down in the guidelines issued by the Ministry of Environment of Forests, Govt of India, vide the Ministry’s No 3 -17/1999-RT dated 05.03.13. In dealing with some of the parts of the course though, the syllabus has been under minor revision to facilitate better understanding of the subjects and to provide their appropriate coverage.