Invasive Non-Native Plants Retain Native Mammal Communities in Novel Ecosystems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Following the Finke: a Modern Expedition Down the River of Time
FOLLOWING THE FINKE FOLLOWING THE FINKE: A MODERN EXPEDITION DOWN THE RIVER OF TIME PART I: TRAVERSING AN ANCIENT LAND DR KATE LEEMING HOPS ON HER CUSTOM-MADE BIKE TO TAKE ON THE AUSTRALIAN INTERIOR. WORDS AND PICS: KATE LEEMING Back in 2004, during my 25,000km Great for the local Aboriginal people and wildlife, unpredictable surfaces requires a similar skill Australian Cycle Expedition (GRACE), in the present day and for eons past. If Uluru set to pedalling over snow. My ‘Following the cycling companion Greg Yeoman and symbolises the nation’s heart, then the Finke Finke River’ expedition therefore would double I camped beside the Finke River near to River, or Larapinta as it is known to the local as a credible expedition in its own right and where it intersects with the Stuart Highway. Arrernte, must surely be its ancient artery. as excellent physical and mental training for We were on our way to Uluru and beyond This is where the germ of my idea to travel cycling across Antarctica. and the Finke River crossing was at the end the course of the Finke River evolved, however The Finke originates about 130km west of of our first day’s ride south of Alice Springs. the concept of biking along the sandy and Alice Springs in the West MacDonnell Ranges, I’d aimed to reach this point because I stony bed of the ephemeral river at that time the remnants of an ancient system of fold wanted to experience camping beside was an impossibility. A decade later, the mountains that was once on the scale of the what is commonly referred to as the world’s development of fatbike technology began Himalayas, but has now diminished to be a oldest river. -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

MORNINGTON PENINSULA BIODIVERSITY: SURVEY and RESEARCH HIGHLIGHTS Design and Editing: Linda Bester, Universal Ecology Services
MORNINGTON PENINSULA BIODIVERSITY: SURVEY AND RESEARCH HIGHLIGHTS Design and editing: Linda Bester, Universal Ecology Services. General review: Sarah Caulton. Project manager: Garrique Pergl, Mornington Peninsula Shire. Photographs: Matthew Dell, Linda Bester, Malcolm Legg, Arthur Rylah Institute (ARI), Mornington Peninsula Shire, Russell Mawson, Bruce Fuhrer, Save Tootgarook Swamp, and Celine Yap. Maps: Mornington Peninsula Shire, Arthur Rylah Institute (ARI), and Practical Ecology. Further acknowledgements: This report was produced with the assistance and input of a number of ecological consultants, state agencies and Mornington Peninsula Shire community groups. The Shire is grateful to the many people that participated in the consultations and surveys informing this report. Acknowledgement of Country: The Mornington Peninsula Shire acknowledges Aboriginal and Torres Strait Islanders as the first Australians and recognises that they have a unique relationship with the land and water. The Shire also recognises the Mornington Peninsula is home to the Boonwurrung / Bunurong, members of the Kulin Nation, who have lived here for thousands of years and who have traditional connections and responsibilities to the land on which Council meets. Data sources - This booklet summarises the results of various biodiversity reports conducted for the Mornington Peninsula Shire: • Costen, A. and South, M. (2014) Tootgarook Wetland Ecological Character Description. Mornington Peninsula Shire. • Cook, D. (2013) Flora Survey and Weed Mapping at Tootgarook Swamp Bushland Reserve. Mornington Peninsula Shire. • Dell, M.D. and Bester L.R. (2006) Management and status of Leafy Greenhood (Pterostylis cucullata) populations within Mornington Peninsula Shire. Universal Ecology Services, Victoria. • Legg, M. (2014) Vertebrate fauna assessments of seven Mornington Peninsula Shire reserves located within Tootgarook Wetland. -

An Investigation Into Factors Affecting Breeding Success in The
An investigation into factors affecting breeding success in the Tasmanian devil (Sarcophilus harrisii) Tracey Catherine Russell Faculty of Science School of Life and Environmental Science The University of Sydney Australia A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy 2018 Faculty of Science The University of Sydney Table of Contents Table of Figures ............................................................................................................ viii Table of Tables ................................................................................................................. x Acknowledgements .........................................................................................................xi Chapter Acknowledgements .......................................................................................... xii Abbreviations ................................................................................................................. xv An investigation into factors affecting breeding success in the Tasmanian devil (Sarcophilus harrisii) .................................................................................................. xvii Abstract ....................................................................................................................... xvii 1 Chapter One: Introduction and literature review .............................................. 1 1.1 Devil Life History ................................................................................................... -

Phylogenetic Structure of Vertebrate Communities Across the Australian
Journal of Biogeography (J. Biogeogr.) (2013) 40, 1059–1070 ORIGINAL Phylogenetic structure of vertebrate ARTICLE communities across the Australian arid zone Hayley C. Lanier*, Danielle L. Edwards and L. Lacey Knowles Department of Ecology and Evolutionary ABSTRACT Biology, Museum of Zoology, University of Aim To understand the relative importance of ecological and historical factors Michigan, Ann Arbor, MI 48109-1079, USA in structuring terrestrial vertebrate assemblages across the Australian arid zone, and to contrast patterns of community phylogenetic structure at a continental scale. Location Australia. Methods We present evidence from six lineages of terrestrial vertebrates (five lizard clades and one clade of marsupial mice) that have diversified in arid and semi-arid Australia across 37 biogeographical regions. Measures of within-line- age community phylogenetic structure and species turnover were computed to examine how patterns differ across the continent and between taxonomic groups. These results were examined in relation to climatic and historical fac- tors, which are thought to play a role in community phylogenetic structure. Analyses using a novel sliding-window approach confirm the generality of pro- cesses structuring the assemblages of the Australian arid zone at different spa- tial scales. Results Phylogenetic structure differed greatly across taxonomic groups. Although these lineages have radiated within the same biome – the Australian arid zone – they exhibit markedly different community structure at the regio- nal and local levels. Neither current climatic factors nor historical habitat sta- bility resulted in a uniform response across communities. Rather, historical and biogeographical aspects of community composition (i.e. local lineage per- sistence and diversification histories) appeared to be more important in explaining the variation in phylogenetic structure. -
1 Early Earth June06.Indd
The Early Earth & First Signs of Life Earth began to solidify and divide into its layers (Core, Mantle and Crust) more than 4 billion years ago – and finally to have a solid surface – unlike Jupiter and Saturn, but more like Mars. But it was not until about 3.8 billion years ago that life is first recorded on Earth by structures called stromatolites “constructed” by bacteria. Their distant relatives are still alive in Australia today, building the same monuments. The early Earth lacked much of an atmosphere and so was heavily pummeled by meteorites. It was a bleak and hellish place, with volcanoes blasting lava fountains in the air, fumeroles steaming – and little water around. But it was this very volcanic activity that formed water and produced the gases which made up an atmosphere, when temperatures on the Earth’s surface finally dipped below 100 o C. – an atmosphere dominated by carbon dioxide, some nitrogen, water vapour, methane and smaller amounts of hydrogen sulfide (which is what makes rotten eggs smell so bad!), hydrogen cyanide and ammonia. There was no significant amount of oxygen in this early atmosphere. By 3800 million years ago there was a solid surface on Earth, and sediments were actually forming – meaning that wind and running water had to be present. How do we know this? Geologists who have studied modern rivers and desert sands, ocean shores and ocean depths compare the sands and muds today with the same sorts of structures (such as ripple marks) and textures preserved in the ancient rocks of the Macdonnell Ranges of Central Australia and the Pilbara region of Western Australia and see many similarities. -

Galahs This Is the Longer Version of an Article to Be Published in Australian Historical Studies in April 2010. Copyright Bill
Galahs This is the longer version of an article to be published in Australian Historical Studies in April 2010. Copyright Bill Gammage, 3 November 2008. Email [email protected] When Europeans arrived in Australia, galahs were typically inland birds, quite sparsely distributed. Now they range from coast to coast, and are common. Why did this change occur? Why didn’t it occur earlier? Galahs feed on the ground. They found Australia’s dominant inland grasses too tall to get at the seed, so relied on an agency to shorten them: Aboriginal grain cropping before contact, introduced stock after it. *** On 3 July 1817, near the swamps filtering the Lachlan to the Murrumbidgee and further inland than any white person had been, John Oxley wrote, ‘Several flocks of a new description of pigeon were seen for the first time... A new species of cockatoo or paroquet, being between both, was also seen, with red necks and breasts, and grey backs. I mention these birds particularly, as they are the only ones we have yet seen which at all differ from those known on the east coast’ [1]. Allan Cunningham, Oxley’s botanist, also saw the birds. ‘We shot a brace of pigeons of a new species...’, he noted, ‘Some other strange birds were observed (supposed to be Parrots), about the size and flight of a pigeon, with beautiful red breasts’, and next morning, ‘They are of a light ash colour on the back and wings, and have rich pink breasts and heads’ [1]. In the manner of science parrot and pigeon were shot, and within a few months John Lewin in Sydney drew the first known depictions of them [53]. -

Alice Springs & Macdonnell Ranges Summary-01.Indd
Destination Management Plan Alice Springs and MacDonnell Ranges Region 2020 Summary Key Partners 1 Front Cover: Trephina Gorge Nature Park – East MacDonnell Ranges Back Cover: Hermannsburg Potters - Ntaria / Hermannsburg This Page: RT Tours2 Australia - Tjoritja / West MacDonnell National Park Contents Destination Management Plan role and process 5 Alice Springs and MacDonnell Ranges Region overview 6 Tourism in the Region Value of tourism in the Region Visitor market profile Trends in regional tourism Destination management planning for the Alice 12 Springs and MacDonnell Ranges Region Guiding principles Destination awareness Approach to developing visitor experiences in the Region Industry gaps and opportunities Action plan 15 Capacity building activities Facilitation of collaborative action Strategic product packaging and marketing Investment attraction initiatives Product development opportunities 19 Implementation 20 Reporting and reviews 22 Acronyms – References – Further information 22 3 Hermannsburg Historic Precinct – Ntaria / Hermannsburg 4 Destination Management Plan role and process The Department of Industry, Destination management requires Tourism and Trade has invested alignment and collaboration across the in destination management public, private and community sectors. It involves stakeholders from both the planning as part of a suite tourism and general industry sectors of actions following the contributing to the development development and release of priority experiences in the Alice of the NT’s Tourism Industry Springs and MacDonnell Ranges Strategy 2030. Destination Region. management ensures that Strategically planned and tourism is cohesively integrated implemented tourism experiences can be an economic driver, contributing into the economic, social, to the growth and development cultural and ecological fabrics of a Region through job creation, of a community, by considering investment attraction, and tourism growth holistically, infrastructure development. -

Uluru & Outback Northern Territory
©Lonely Planet Publications Pty Ltd Uluru & Outback Northern Territory Includes ¨ Why Go? KATHERINE. 181 The remote and largely untamed chunk of the Northern BARKLY TABLELAND Territory from Katherine to Uluru is where dreams end and & GULF COUNTRY ... 191 adventure begins. If you enjoy off-road driving, meeting real CENTRAL NT .......194 characters of the Australian outback, and contortions of an Tennant Creek. .195 ancient land sliced into escarpments, canyons, gorges and pockets of verdant bush, then you’ve come to the right place. Tanami Track ........198 The Stuart Hwy from Katherine to Alice Springs is still re- ALICE SPRINGS .....198 ferred to as ‘the Track’ – it has been since WWII, when it was MACDONNELL literally a dirt track connecting the NT’s two main towns. It’s RANGES ...........208 dead straight most of the way. The Red Centre is Australia’s NT’S FAR SOUTH ....214 heartland, boasting the iconic attractions of Uluru (Ayers KINGS CANYON .....216 Rock) and Kata Tjuta (The Olgas), plus an enigmatic Cen- tral Desert culture that continues to produce extraordinary ULURU-KATA TJUTA NATIONAL PARK ....217 abstract art. And delighting travellers with its eccentric of- ferings, pioneering spirit and weathered mountain setting, Alice Springs is the city at the centre of a continent. Best Places to Eat ¨ Marksies Camp Tucker When to Go (p183) Alice Springs ¨ Sounds of Silence (p218) °C/°F Te mp Rainfall inches/mm ¨ Black Russian Caravan Bar 40/104 8/200 (p183) 30/86 6/150 ¨ Montes (p204) 20/68 4/100 Best Places 10/50 2/50 0/32 0 to Sleep J FDM A M J J A S O N ¨ Desert Gardens Hotel (p219) Apr–Aug Peak Jun & Jul Festival Sep–Mar It’s hot, season with cooler season: Beanie hot, hot around ¨ Lorella Springs Wilderness temperatures in Festival and Alice Springs; Park (p195) the Red Centre. -
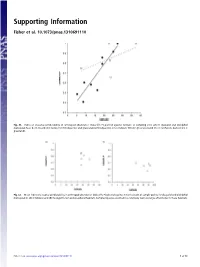
Supporting Information
Supporting Information Fisher et al. 10.1073/pnas.1310691110 Fig. S1. Index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against latitude of sampling sites where dasyurid and didelphid marsupials have been recorded in rainforest (filled points) and grassland (unfilled points). Lines indicate fitted regressions (solid line = rainforest, dashed line = grassland). Fig. S2. Mean index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against mean latitude of sample points for dasyurid and didelphid marsupials in (A) shrubland and (B) Eucalypt forest and woodland habitats. Sampled species occurred in a relatively narrow range of latitudes in these habitats. Fisher et al. www.pnas.org/cgi/content/short/1310691110 1of13 Fig. S3. Phylogeny of insectivorous marsupials with known life history data, based on ref. 1 with updates from ref. 2. 1. Cardillo M, Bininda-Emonds ORP, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264(1):11–31. 2. Fritz SA, Bininda-Emonds ORP, Purvis A (2009) Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol Lett 12(6):538–549. Fisher et al. www.pnas.org/cgi/content/short/1310691110 2of13 Fisher et al. Table S1. Reproductive traits and diets of insectivorous marsupials Latitude (south) www.pnas.org/cgi/content/short/1310691110 Proportion Proportion Female for Habitat of of age species class for females males Male Breeding Copulation Litters Scrotal at first with species Genus species -

Draft National Recovery Plan for the Central Rock-Rat Zyzomys Pedunculatus
National Recovery Plan for the Central Rock-rat Zyzomys pedunculatus © Department of Environment and Natural Resources, Northern Territory This work is copyright. It may be reproduced for study, research or training purposes subject to an acknowledgment of the sources but no commercial usage or sale. Requests and enquiries concerning reproduction and rights should be addressed to: Director, Species Conservation Flora and Fauna Division Dept. of Environment and Natural Resources Northern Territory Government PO Box 1120 ALICE SPRINGS NT 0871. This recovery plan sets out the actions necessary to stop the decline of, and support the recovery of, the listed threatened species. The Australian Government is committed to acting in accordance with the plan and to implementing the plan as it applies to Commonwealth areas. The plan has been developed with the involvement and cooperation of a broad range of stakeholders, but individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge. This plan should be cited as follows: McDonald P, Ward S, Nano C, Pavey C, Nano T, Nunn P (2017). National Recovery Plan for the Central Rock-rat, Zyzomys pedunculatus. Northern Territory Dept. of Environment and Natural Resources. Alice Springs, Northern Territory. Copies of the plan are available at: http://www.environment.gov.au/biodiversity/threatened/recovery-list-common.html TABLE OF CONTENTS TABLE OF CONTENTS 1 ABBREVIATIONS 3 SUMMARY 4 1. -

Submission by the Friends of Eastern Otways (Great Otway National Park
Submission by The Friends of Eastern Otways (Great Otway National Park) Incorporated [FEO (GONP)] to the Environment and Communications References Senate Committee on Faunal Extinction The FEO (GONP) is a community organisation of over 200 members whose area of operation is the Eastern area of the Great Otway National Park. The Friends carry out environmental care activities including weeding, regeneration of native vegetation and flora and fauna monitoring, in collaboration with Parks Victoria. Since 1998, FEO (GONP) has undertaken fauna monitoring in the Eastern Otways. Initially (1998 -2008) hair tubes were employed as animals enter the baited tube they leave hair samples and the animal is identified after analysis of the hair structure. More recently (2009 to 2017) remote, infra-red sensing cameras with attractants to draw native fauna to the camera sites have replaced hair tubes. With camera monitoring, the resultant images enable direct and immediate identification of a wide range of animal species. In the years since we commenced fauna monitoring we have carried out many thousands of hours of monitoring and identified many species of fauna in the area of the Eastern Otways (stretching from Point Addis in the East through Anglesea, Aireys Inlet and Lorne to Kennett River in the west, including the coastal hinterland). We make this submission to draw the committee’s attention to the decline in the number and variety of faunal species we have obtained using our monitoring methods. We would particularly wish to draw the Committee’s attention to the decline in small, native, terrestrial, mammals we have observed. Australia has many small terrestrial mammals: They are important in ecosystems such as found in GONP.