Autism Spectrum Disorders Veatch OJ, Veenstra-Vanderweele J, Potter M, Pericak-Vance MA, Haines JL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
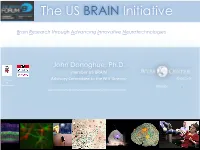
The US BRAIN Initiative
The US BRAIN Initiative Brain Research through Advancing Innovative Neurotechnologies John Donoghue, Ph.D. member US BRAIN Geneva Professor Advisory Commi5ee to the NIH Director of Neuroscience Director 1 views expressed here are my own and not those of the US Government Why a US BRAIN Initiative? The Need Is Great § Brain disorders: #1 source of disability in U.S. – > 100 million Americans affected § Rates are increasing § Costs are increasing- § annual cost of dementia ~$200B § by 2050 >$1Trillion § Already equals cost of cancer and heart disease The cost of disorders of the brain in Europe amounts overall to €798 billion in 2010 Eur Neuropsych. (2011) 21, 817-779 WHO, 2008 The Challenge for the 21st Century We do NOT know enough about the brain source: Donoghue! 3 “The Next Great American Project” “So there is this enormous mystery waiPng to be unlocked, and the BRAIN IniPave will change that by giving sciensts the tools they need to get a dynamic picture of the brain in acCon and be5er understand how we think and how we learn and how we remember. And that knowledge could be – will be – transformave.” -- President Obama, April 2, 2013 4 US BRAIN Initiative “a public and private effort” + Private Investments 5 The NIH BRAIN ScienCfic Plan • NIH Advisory Committee to the Director- William Newsome, PhD (co-chair) Working Group Stanford University (scientists, federal officials) • Met 2013-14 • Broad input from Cornelia Bargmann, PhD scientific community (co-chair) The Rockefeller University • Released June 2014 FIRST FIVE YEARS SECOND FIVE YEARS Emphasize technology Emphasize discovery development driven science 6 Understanding the Brain as a SYSTEM To map the circuits of the brain, measure the fluctuang paerns of electrical and chemical acPvity flowing within those circuits, and understand how their interplay creates our unique cogniPve and behavioral capabiliPes. -
![Reviewers [PDF]](https://docslib.b-cdn.net/cover/7014/reviewers-pdf-667014.webp)
Reviewers [PDF]
The Journal of Neuroscience, January 2013, 33(1) Acknowledgement For Reviewers 2012 The Editors depend heavily on outside reviewers in forming opinions about papers submitted to the Journal and would like to formally thank the following individuals for their help during the past year. Kjersti Aagaard Frederic Ambroggi Craig Atencio Izhar Bar-Gad Esther Aarts Céline Amiez Coleen Atkins Jose Bargas Michelle Aarts Bagrat Amirikian Lauren Atlas Steven Barger Lawrence Abbott Nurith Amitai David Attwell Cornelia Bargmann Brandon Abbs Yael Amitai Etienne Audinat Michael Barish Keiko Abe Martine Ammasari-Teule Anthony Auger Philip Barker Nobuhito Abe Katrin Amunts Vanessa Auld Neal Barmack Ted Abel Costas Anastassiou Jesús Avila Gilad Barnea Ute Abraham Beau Ances Karen Avraham Carol Barnes Wickliffe Abraham Richard Andersen Gautam Awatramani Steven Barnes Andrey Abramov Søren Andersen Edward Awh Sue Barnett Hermann Ackermann Adam Anderson Cenk Ayata Michael Barnett-Cowan David Adams Anne Anderson Anthony Azevedo Kevin Barnham Nii Addy Clare Anderson Rony Azouz Scott Barnham Arash Afraz Lucy Anderson Hiroko Baba Colin J. Barnstable Ariel Agmon Matthew Anderson Luiz Baccalá Scott Barnum Adan Aguirre Susan Anderson Stephen Baccus Ralf Baron Geoffrey Aguirre Anuska Andjelkovic Stephen A. Back Pascal Barone Ehud Ahissar Rodrigo Andrade Lars Bäckman Maureen Barr Alaa Ahmed Ole Andreassen Aldo Badiani Luis Barros James Aimone Michael Andres David Badre Andreas Bartels Cheryl Aine Michael Andresen Wolfgang Baehr David Bartés-Fas Michael Aitken Stephen Andrews Mathias Bähr Alison Barth Elias Aizenman Thomas Andrillon Bahador Bahrami Markus Barth Katerina Akassoglou Victor Anggono Richard Baines Simon Barthelme Schahram Akbarian Fabrice Ango Jaideep Bains Edward Bartlett Colin Akerman María Cecilia Angulo Wyeth Bair Timothy Bartness Huda Akil Laurent Aniksztejn Victoria Bajo-Lorenzana Marisa Bartolomei Michael Akins Lucio Annunziato David Baker Marlene Bartos Emre Aksay Daniel Ansari Harriet Baker Jason Bartz Kaat Alaerts Mark S. -

3 and 4/1998 Genetic Engineering and Biotechnology
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] EMERGING TECHNOLOGY SERIES 3 and 4/1998 Genetic Engineering and Biotechnology UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna, 1999 TO OUR READERS EMERGING TECHNOLOGY SERIES: Globalization, the information society, sustainable development: these are the keywords of the day. -

Dynamics of Excitatory-Inhibitory Neuronal Networks With
I (X;Y) = S(X) - S(X|Y) in c ≈ p + N r V(t) = V 0 + ∫ dτZ 1(τ)I(t-τ) P(N) = 1 V= R I N! λ N e -λ www.cosyne.org R j = R = P( Ψ, υ) + Mγ (Ψ, υ) σ n D +∑ j n k D k n MAIN MEETING Salt Lake City, UT Feb 27 - Mar 2 ................................................................................................................................................................................................................. Program Summary Thursday, 27 February 4:00 pm Registration opens 5:30 pm Welcome reception 6:20 pm Opening remarks 6:30 pm Session 1: Keynote Invited speaker: Thomas Jessell 7:30 pm Poster Session I Friday, 28 February 7:30 am Breakfast 8:30 am Session 2: Circuits I: From wiring to function Invited speaker: Thomas Mrsic-Flogel; 3 accepted talks 10:30 am Session 3: Circuits II: Population recording Invited speaker: Elad Schneidman; 3 accepted talks 12:00 pm Lunch break 2:00 pm Session 4: Circuits III: Network models 5 accepted talks 3:45 pm Session 5: Navigation: From phenomenon to mechanism Invited speakers: Nachum Ulanovsky, Jeffrey Magee; 1 accepted talk 5:30 pm Dinner break 7:30 pm Poster Session II Saturday, 1 March 7:30 am Breakfast 8:30 am Session 6: Behavior I: Dissecting innate movement Invited speaker: Hopi Hoekstra; 3 accepted talks 10:30 am Session 7: Behavior II: Motor learning Invited speaker: Rui Costa; 2 accepted talks 11:45 am Lunch break 2:00 pm Session 8: Behavior III: Motor performance Invited speaker: John Krakauer; 2 accepted talks 3:45 pm Session 9: Reward: Learning and prediction Invited speaker: Yael -

As the Worm Turns with the Help of a Tiny Worm, Cornelia Bargmann Is Unpicking the Neural Circuits That Drive Eating, Socializing and Sex
NEWS FEATURE As the worm turns With the help of a tiny worm, Cornelia Bargmann is unpicking the neural circuits that drive eating, socializing and sex. BY STEPHEN S. HALL ale sexual dysfunction is never pretty, even in nematodes. every animal has to show and every animal has to solve?” she asks. “You In normal roundworm courtship, a slender male will sidle can basically say that the three would be hunger, fear and reproduction. up to a plump hermaphrodite, make contact, and then ini- None of those things got invented last Saturday night!” tiate a set of steps leading up to insemination: a sinuous The evolutionary strength of that argument grew last year, when Mbackwards motion as he searches for the sexual cleft, a pause to probe, Bargmann and her colleagues published the experiments with the and finally the transfer of sperm. The whole business is usually over in mutant males1. The mutation, they reported, is in a roundworm gene a couple of minutes. “It’s very slithery, and affectionate,” says Cornelia that they dubbed nematocin, which codes for a small peptide that influ- Bargmann, who has been observing the behaviour of this particular ences multiple neurons and is a biochemical cousin to oxytocin and vas- worm, Caenorhabditis elegans, for 25 years. opressin, two hormones that play key parts in mammalian reproductive Last October, scientists in Bargmann’s laboratory at the Rockefeller behaviour. Put another, Darwinian, way, the sexual confusion in mutant University, New York, reported the discovery of a gene that seems to be nematode males is tied to a molecule that seems to have been conserved crucial to successful mating. -

Biography of Cornelia I. Bargmann BIOGRAPHY
Corrections MEDICAL SCIENCES. For the article ‘‘HLA-B*5801 allele as a BIOGRAPHY, NEUROSCIENCE. For the article ‘‘Biography of Cornelia genetic marker for severe cutaneous adverse reactions caused by I. Bargmann,’’ by Melissa Marino, which appeared in issue 9, allopurinol,’’ by Shuen-Iu Hung, Wen-Hung Chung, Lieh-Bang March 1, 2005, of Proc. Natl. Acad. Sci. USA (102, 3181–3183; Liou, Chen-Chung Chu, Marie Lin, Hsien-Ping Huang, Yen- first published February 22, 2005; 10.1073͞pnas.0500025102), Ling Lin, Joung-Liang Lan, Li-Cheng Yang, Hong-Shang Hong, due to an editorial office error, the term ‘‘roundworm’’ was Ming-Jing Chen, Ping-Chin Lai, Mai-Szu Wu, Chia-Yu Chu, incorrectly replaced with ‘‘flatworm’’ in the first sentence. The Kuo-Hsien Wang, Chien-Hsiun Chen, Cathy S. J. Fann, Jer- correct sentence should read as follows: ‘‘In the unhearing, Yuarn Wu, and Yuan-Tsong Chen, which appeared in issue 11, unseeing world of the roundworm Caenorhabditis elegans, its March 15, 2005, of Proc. Natl. Acad. Sci. USA (102, 4134–4139; sense of smell is its lifeline.’’ first published March 2, 2005; 10.1073͞pnas.0409500102), due to ͞ ͞ ͞ ͞ a printer’s error, the symbols in the affiliation line appeared www.pnas.org cgi doi 10.1073 pnas.0502717102 incorrectly. The corrected affiliation line appears below. aInstitute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan; Departments of cDermatology, eRheumatology, Allergy, and Immunology, and hNephrology, Chang Gung Memorial Hospital, Taipei 10507, Taiwan; fDepartment of Medical Research, -

C. Elegans Avoidance Behavior
The genetic, neuronal, and chemical basis for microbial discrimination in Caenorhabditiselegans by MASSACHUSETTS INSTITUTE Of TECHNOLOGY Joshua D. Meisel MAY 252016 M.S. Biology, B.S. Biology, B.A. English Stanford University, 2007 LIBRARIES MRCHP/E SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2016 2016 Joshua D. Meisel. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature redacted Signature of Author: Department of Biology May 20, 2016 Certified by: Signature redacted Dennis H. Kim Associate Professor of Biology A Thesis Supervisor Accepted by: Signature redacted 7 Amy E. Keating Professor of Biology Co-Chair, Biology Graduate Committee The genetic, neuronal, and chemical basis for microbial discrimination in Caenorhabditiselegans by Joshua D. Meisel Submitted to the Department of Biology on May 20, 2016 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology ABSTRACT Discrimination among pathogenic and beneficial microbes is essential for host organism immunity and homeostasis. Increasingly, the nervous system of animals is being recognized as an important site of bacterial recognition, but the molecular mechanisms underlying this process remain unclear. Chapter One discusses how the nematode Caenorhabditiselegans can be used to dissect the genetic and neuronal mechanisms that coordinate behavioral responses to bacteria. In Chapter Two, we show that chemosensory detection of two secondary metabolites produced by Pseudomonas aeruginosamodulates a neuroendocrine signaling pathway that promotes C. -

April 8-11, 2019 the 2019 Franklin Institute Laureates the 2019 Franklin Institute AWARDS CONVOCATION APRIL 8–11, 2019
april 8-11, 2019 The 2019 Franklin Institute Laureates The 2019 Franklin Institute AWARDS CONVOCATION APRIL 8–11, 2019 Welcome to The Franklin Institute Awards, the range of disciplines. The week culminates in a grand oldest comprehensive science and technology medaling ceremony, befitting the distinction of this awards program in the United States. Each year, the historic awards program. Institute recognizes extraordinary individuals who In this convocation book, you will find a schedule of are shaping our world through their groundbreaking these events and biographies of our 2019 laureates. achievements in science, engineering, and business. We invite you to read about each one and to attend We celebrate them as modern day exemplars of our the events to learn even more. Unless noted otherwise, namesake, Benjamin Franklin, whose impact as a all events are free and open to the public and located scientist, inventor, and statesman remains unmatched in Philadelphia, Pennsylvania. in American history. Along with our laureates, we honor Franklin’s legacy, which has inspired the We hope this year’s remarkable class of laureates Institute’s mission since its inception in 1824. sparks your curiosity as much as they have ours. We look forward to seeing you during The Franklin From shedding light on the mechanisms of human Institute Awards Week. memory to sparking a revolution in machine learning, from sounding the alarm about an environmental crisis to making manufacturing greener, from unlocking the mysteries of cancer to developing revolutionary medical technologies, and from making the world III better connected to steering an industry giant with purpose, this year’s Franklin Institute laureates each reflect Ben Franklin’s trailblazing spirit. -
Promise, Problems
20110822-NEWS--0001-NAT-CCI-CN_-- 8/19/2011 6:59 PM Page 1 INSIDE NEXT WEEK 9/11 TOP STORIES 10 YEARS Coney LATER Island’s Plus: Our annual biggest ® CITY FACTS thrills still to come double issue PAGE 3 August in New York: VOL. XXVII, NO. 34 WWW.CRAINSNEWYORK.COM AUGUST 22-28, 2011 PRICE: $3.00 why it’s city’s most surprising month MICHAEL GROSS, PAGE 2 Bank of Pro soccer America is back in town—but breakup maybe not for long, unless may loom fans turn up NEIGHBORHOOD JOURNAL, Loss outlook staggers PAGE 2 investors, who say One clear winner in BofA should return Verizon vs. unions: to its regional roots Time Warner Cable BY AARON ELSTEIN NEW YORK, NEW YORK, P. 6 Shortly before the turn of the millen- nium, when Bank of America assem- bled the first nationwide banking fran- chise, it unveiled a red, white and blue logo designed to evoke the American flag and amber waves of grain. Today, the logo emphasizes only the coast-to- coast mess the country’s largest bank finds itself in. “You can boil down Bank of Amer- ica’s difficulties to this: It is the bank to America,” said Nancy Bush, a banking analyst at NAB Research. “The na- tion’s problems are its problems.” BUSINESS LIVES With the domestic economy seem- ingly grinding to a halt and housing See BANK OF AMERICA on Page 24 GOTHAM GIGS Broadway’s dialect coach P. 25 ● ANNE FISHER Reinventing the bonus plan pays off P. 25 Food push: ● MOVERS & SHAKERS Pushing Condé Nast BRAIN POWER into the digital age P. -

Amplification of the Proto-Neu Oncogene Facilitates Oncogenic
Proc. NatI. Acad. Sci. USA Vol. 86, pp. 2545-2548, April 1989 Biochemistry Amplification of the proto-neu oncogene facilitates oncogenic activation by a single point mutation (gene amplification/multiple-step carcinogenesis) MIEN-CHIE HUNG, DUEN-HWA YAN, AND XIAOYAN ZHAO Department of Tumor Biology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030 Communicated by Robert A. Weinberg, December 13, 1988 ABSTRACT To determine whether the amplification of encoded p185 protein (13, 14) but exhibit normal morphology the proto-neu oncogene (also called c-erbB-2) plays a role in and form monolayers when growing to confluency (15). tumorigenicity, we previously generated an NIH 3T3 transfec- We studied the tumorigenicity of the DHFR/G-8 cells and tant (DHFR/G-8) that carried the amplified proto-neu gene. found that amplification of the proto-neu gene in DHFR/G-8 The DHFR/G-8 cells exhibited normal morphology. Their cells facilitated a single point mutation that rendered the cells growth curve was similar to that of NIH 3T3 cells but was tumorigenic. The results have important implications in the different from that of the B104-1 cell, an NIH 3T3 transfectant pathogenesis of human tumors-namely, the amplified pro- that carries the activated neu oncogene. When injected into tooncogene in human tumors could be activated by convert- nude mice, B104-1 cells produced tumors within 2 weeks, ing one (or a small number) of the amplified protooncogenes whereas the DHFR/G-8 cells did not produce tumors until 3 into an activated oncogene in addition to producing a high months after injection, and the NIH 3T3 cells did not produce level of protooncogene protein product. -

Results of the 2014 Sfn Election What's Ahead in Careers In
SUMMER 2014 Message from the President What’s Ahead in Careers in Neuroscience It is truly the golden age of neuroscience. Advances in technology “Let your love of science be are ushering in opportunities beyond our wildest dreams, enabling us to trace the connections between brain regions, probe brain your guide as you navigate function with light, and connect genes with cell- and systems- a career path. Pursue your wide neural processes. Progress is being made at a dazzling speed, and neuroscience is in the limelight thanks to the BRAIN goals, show passion for them, Initiative, the Human Brain Project, and other research ventures and do everything in your around the world. Carol Mason, power to attain your dreams.” SfN President As a co-director of Columbia University’s neurobiology graduate program, I and my colleagues at other institutions have witnessed a sea change in the career paths of trainees in recent years. For graduate students and – Carol Mason, postdocs now — those who haven’t started to think about careers and those who have SfN President done all that’s recommended but are frustrated by their efforts — the thrill of continuing in neuroscience is offset by worry about the future. Can I succeed in a ramped-up compe- tition for funding and publishing that shows little sign of abating? Will I find a position in the same city as my mate? Is it possible to be a scientist in such a harsh environment and still have a life? Will I be able to land an academic position or NIH grant? And if I choose to go off the academic track, intentionally or reluctantly, what career choices are out there? IN THIS ISSUE Continued on page 4 … Message from the President ............................... -

Oral History Center University of California the Bancroft Library Berkeley, California
Oral History Center, The Bancroft Library, University of California Berkeley Oral History Center University of California The Bancroft Library Berkeley, California J. Michael Bishop Scientist, UCSF Chancellor, and Nobel Laureate Interviews conducted by Sally Smith Hughes in 2016 and 2017 Copyright © 2017 by The Regents of the University of California Oral History Center, The Bancroft Library, University of California Berkeley ii Since 1954 the Oral History Center of the Bancroft Library, formerly the Regional Oral History Office, has been interviewing leading participants in or well-placed witnesses to major events in the development of Northern California, the West, and the nation. Oral History is a method of collecting historical information through tape-recorded interviews between a narrator with firsthand knowledge of historically significant events and a well-informed interviewer, with the goal of preserving substantive additions to the historical record. The tape recording is transcribed, lightly edited for continuity and clarity, and reviewed by the interviewee. The corrected manuscript is bound with photographs and illustrative materials and placed in The Bancroft Library at the University of California, Berkeley, and in other research collections for scholarly use. Because it is primary material, oral history is not intended to present the final, verified, or complete narrative of events. It is a spoken account, offered by the interviewee in response to questioning, and as such it is reflective, partisan, deeply involved, and irreplaceable. ********************************* All uses of this manuscript are covered by a legal agreement between The Regents of the University of California and J. Michael Bishop dated June 22, 2017. The manuscript is thereby made available for research purposes.