Conditioned Effects of Heroin on the Expression of Inducible Nitric Oxide Synthase in the Rat Are Susceptible to Extinction and Latent Inhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

Medications to Treat Opioid Use Disorder Research Report
Research Report Revised Junio 2018 Medications to Treat Opioid Use Disorder Research Report Table of Contents Medications to Treat Opioid Use Disorder Research Report Overview How do medications to treat opioid use disorder work? How effective are medications to treat opioid use disorder? What are misconceptions about maintenance treatment? What is the treatment need versus the diversion risk for opioid use disorder treatment? What is the impact of medication for opioid use disorder treatment on HIV/HCV outcomes? How is opioid use disorder treated in the criminal justice system? Is medication to treat opioid use disorder available in the military? What treatment is available for pregnant mothers and their babies? How much does opioid treatment cost? Is naloxone accessible? References Page 1 Medications to Treat Opioid Use Disorder Research Report Discusses effective medications used to treat opioid use disorders: methadone, buprenorphine, and naltrexone. Overview An estimated 1.4 million people in the United States had a substance use disorder related to prescription opioids in 2019.1 However, only a fraction of people with prescription opioid use disorders receive tailored treatment (22 percent in 2019).1 Overdose deaths involving prescription opioids more than quadrupled from 1999 through 2016 followed by significant declines reported in both 2018 and 2019.2,3 Besides overdose, consequences of the opioid crisis include a rising incidence of infants born dependent on opioids because their mothers used these substances during pregnancy4,5 and increased spread of infectious diseases, including HIV and hepatitis C (HCV), as was seen in 2015 in southern Indiana.6 Effective prevention and treatment strategies exist for opioid misuse and use disorder but are highly underutilized across the United States. -

National Vital Statistics Report: Drugs Most Frequently Involved In
National Vital Statistics Reports Volume 67, Number 9 December 12, 2018 Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2011–2016 by Holly Hedegaard, M.D., M.S.P.H., and Brigham A. Bastian, B.S., National Center for Health Statistics; James P. Trinidad, M.P.H., M.S., U.S. Food and Drug Administration; Merianne Spencer, M.P.H., and Margaret Warner, Ph.D., National Center for Health Statistics Abstract overdose deaths involving methadone decreased from 1.4 per 100,000 in 2011 to 1.1 in 2016. The 10 most frequently Objective—This report identifies the specific drugs involved mentioned drugs often were found in combination with each most frequently in drug overdose deaths in the United States other. The drugs most frequently mentioned varied by the intent from 2011 through 2016. of the drug overdose death. In 2016, the drugs most frequently Methods—Record-level data from the 2011–2016 National mentioned in unintentional drug overdose deaths were fentanyl, Vital Statistics System–Mortality files were linked to electronic heroin, and cocaine, while the drugs most frequently mentioned files containing literal text information from death certificates. in suicides by drug overdose were oxycodone, diphenhydramine, Drug overdose deaths were identified using the International hydrocodone, and alprazolam. Classification of Diseases, Tenth Revision underlying cause- Conclusions—This report identifies patterns in the specific of-death codes X40–X44, X60–X64, X85, and Y10–Y14. Drug drugs most frequently involved in drug overdose deaths from mentions were identified by searching the literal text in three 2011 through 2016 and highlights the importance of complete fields of the death certificate: the causes of death from Part I, and accurate reporting in the literal text on death certificates. -

What Are the Treatments for Heroin Addiction?
How is heroin linked to prescription drug abuse? See page 3. from the director: Research Report Series Heroin is a highly addictive opioid drug, and its use has repercussions that extend far beyond the individual user. The medical and social consequences of drug use—such as hepatitis, HIV/AIDS, fetal effects, crime, violence, and disruptions in family, workplace, and educational environments—have a devastating impact on society and cost billions of dollars each year. Although heroin use in the general population is rather low, the numbers of people starting to use heroin have been steadily rising since 2007.1 This may be due in part to a shift from abuse of prescription pain relievers to heroin as a readily available, cheaper alternative2-5 and the misperception that highly pure heroin is safer than less pure forms because it does not need to be injected. Like many other chronic diseases, addiction can be treated. Medications HEROIN are available to treat heroin addiction while reducing drug cravings and withdrawal symptoms, improving the odds of achieving abstinence. There are now a variety of medications that can be tailored to a person’s recovery needs while taking into account co-occurring What is heroin and health conditions. Medication combined with behavioral therapy is particularly how is it used? effective, offering hope to individuals who suffer from addiction and for those around them. eroin is an illegal, highly addictive drug processed from morphine, a naturally occurring substance extracted from the seed pod of certain varieties The National Institute on Drug Abuse (NIDA) has developed this publication to Hof poppy plants. -

Adolescent Drug Terminology and Trends: 2017-18 Edition
Adolescent Drug Terminology and Trends: 2017-18 Edition Matthew Quinn, LCPC, CADC Community Relations Coordinator Vaping Term used to describe when a substance is heated to the point of releasing vapor but not combusted (lit on fire). • Increasing in popularity as a way to ingest nicotine and cannabis, often in an electronic device that looks like a pen • Usually relatively odorless and difficult to distinguish between nicotine and cannabis vape device Juul (pronounced jewel) Specific vaping product from Pax Labs similar to an e- cigarette used to ingest nicotine • Liquid contains nicotine salts extracted from the tobacco leaf (2x nicotine of previous e-cigs) • Variety of flavors • Cool mint • Mango • Crème brule Dabs Dabs is a highly concentrated butane hash oil (BHO) created in a process where high quality cannabis is blasted with butane and extracted. • Heated and inhaled • Contains 70-90% THC compared to 5-15% THC in regular cannabis • Wax, oil, shatter, crumble • Sauce, distillate Rig A rig is a device used to vaporize and inhale dabs. • Looks similar to a water pipe or bong • Usually a nail is heated with a hand- held torch to a high temperature and a small piece of the concentrate is ‘dabbed’ onto a nail • Vapor released is then inhaled through the pipe Edibles • Increasingly popular alternative to smoking marijuana • Produced to infuse marijuana into various ingestible forms • Problem is that effects are hard to predict and difficult to know dose Other Terms for Cannabis • Bud • Dank • Nug • Loud • Fire • Gas Bars (Ladders) Another name for the rectangular shaped Xanax (anti- anxiety medication) with three lines in them (typically 2mg per ‘bar’). -
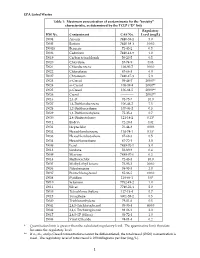
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Drug Fact Sheet: Oxycodone
Oxycodone WHAT IS OXYCODONE? What is its effect on the mind? Oxycodone is a semi-synthetic narcotic analgesic Euphoria and feelings of relaxation are the most and historically has been a popular drug of abuse common effects of oxycodone on the brain, which among the narcotic abusing population. explains its high potential for abuse. WHAT IS ITS ORIGIN? What is its effect on the body? Oxycodone is synthesized from thebaine, a Physiological effects of oxycodone include: constituent of the poppy plant. • Pain relief, sedation, respiratory depression, constipation, papillary constriction, and cough What are common street names? suppression. Extended or chronic use of oxycodone Common street names include: containing acetaminophen may cause severe liver • Hillbilly Heroin, Kicker, OC, Ox, Roxy, Perc, and Oxy damage What does it look like? What are its overdose effects? Oxycodone is marketed alone as OxyContin® in Overdose effects include: 10, 20, 40 and 80 mg extended-release tablets • Extreme drowsiness, muscle weakness, confusion, cold and other immediate-release capsules like 5 and clammy skin, pinpoint pupils, shallow breathing, mg OxyIR®. It is also marketed in combination slow heart rate, fainting, coma, and possible death products with aspirin such as Percodan® or acetaminophen such as Roxicet®. Which drugs cause similar effects? Drugs that cause similar effects to Oxycodone How is it abused? include: Oxycodone is abused orally or intravenously. • Opium, codeine, heroin, methadone, hydrocodone, The tablets are crushed and sniffed or dissolved fentanyl, and morphine in water and injected. Others heat a tablet that has been placed on a piece of foil then inhale the What is its legal status in the United States? vapors. -

NIDA Modified ASSIST (Pdf)
1 NIDA Quick Screen V1.0F Name: ......................................................................... Sex ( ) F ( ) M Age....... Interviewer........................................ Date ....../....../...... Introduction (Please read to patient) Hi, I’m __________, nice to meet you. If it’s okay with you, I’d like to ask you a few questions that will help me give you better medical care. The questions relate to your experience with alcohol, cigarettes, and other drugs. Some of the substances we’ll talk about are prescribed by a doctor (like pain medications). But I will only record those if you have taken them for reasons or in doses other than prescribed. I’ll also ask you about illicit or illegal drug use––but only to better diagnose and treat you. Instructions: For each substance, mark in the appropriate column. For example, if the patient has used cocaine monthly in the past year, put a mark in the “Monthly” column in the “illegal drug” row. NIDA Quick Screen Question: In the past year, how often have you used the following? Daily Twice Never Almost Almost Weekly Daily or Once or Monthly Alcohol • For men, 5 or more drinks a day • For women, 4 or more drinks a day Tobacco Products Prescription Drugs for Non-Medical Reasons Illegal Drugs . If the patient says “NO” for all drugs in the Quick Screen, reinforce abstinence. Screening is complete. If the patient says “Yes” to one or more days of heavy drinking, patient is an at-risk drinker. Please see NIAAA website “How to Help Patients Who Drink Too Much: A Clinical Approach” http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm, for information to Assess, Advise, Assist, and Arrange help for at risk drinkers or patients with alcohol use disorders . -

Hallucinogens
Hallucinogens What are hallucinogens? Hallucinogens are a diverse group of drugs that alter a person’s awareness of their surroundings as well as their own thoughts and feelings. They are commonly split into two categories: classic hallucinogens (such as LSD) and dissociative drugs (such as PCP). Both types of hallucinogens can cause hallucinations, or sensations and images that seem real though they are not. Additionally, dissociative drugs can cause users to feel out of control or disconnected from their body and environment. Some hallucinogens are extracted from plants or mushrooms, and some are synthetic (human- made). Historically, people have used hallucinogens for religious or healing rituals. More recently, people report using these drugs for social or recreational purposes, including to have fun, deal with stress, have spiritual experiences, or just to feel different. Common classic hallucinogens include the following: • LSD (D-lysergic acid diethylamide) is one of the most powerful mind-altering chemicals. It is a clear or white odorless material made from lysergic acid, which is found in a fungus that grows on rye and other grains. LSD has many other street names, including acid, blotter acid, dots, and mellow yellow. • Psilocybin (4-phosphoryloxy-N,N- dimethyltryptamine) comes from certain types of mushrooms found in tropical and subtropical regions of South America, Mexico, and the United States. Some common names for Blotter sheet of LSD-soaked paper squares that users psilocybin include little smoke, magic put in their mouths. mushrooms, and shrooms. Photo by © DEA • Peyote (mescaline) is a small, spineless cactus with mescaline as its main ingredient. Peyote can also be synthetic. -

Surveillance of Drug Abuse Trends in the State of Ohio January - June 2019
OSAMOSAM OhioOhio Substance Substance Abuse Abuse Monitoring Monitoring Network Network SurveillanceSurveillance of of Drug Drug Abuse Abuse Trends Trends in the State of Ohio January - June 2019 Lake Ashtabula Fulton Lucas Williams Ottawa Geauga Cuyahoga Defiance Henry Wood Sandusky Erie Lorain Trumbull Huron Summit Portage Paulding Seneca Medina Putnam Hancock Mahoning Van Wert Wyandot Crawford Ashland Wayne Stark Columbiana Allen Richland Hardin Marion Mercer Auglaize Holmes Carroll Morrow Tuscarawas Jefferson Logan Knox Shelby Union Delaware Coshocton Harrison Champaign Darke Licking Miami Guernsey Belmont Franklin Muskingum Clark Madison Montgomery Preble Fairfield Perry Noble Monroe Greene Pickaway Fayette Morgan Hocking Washington Butler Warren Clinton Ross Athens Vinton Hamilton Highland Clermont Pike Meigs Jackson Brown Adams Scioto Gallia Lawrence Legend Akron-Canton region Columbus region Athens region Dayton region Cincinnati region Toledo region Cleveland region Youngstown region Ohio Substance Abuse Monitoring Network Surveillance of Drug Abuse Trends in the State of Ohio January - June 2019 Prepared by: Ohio Department of Mental Health and Addiction Services Office of Quality, Planning and Research R. Thomas Sherba, OSAM Principal Investigator — PhD, MPH, LPCC Sarah Balser, OSAM Coordinator — MPH, MSW, LSW, CHES Jessica Linley, OSAM Quantitative Data Analyst — PhD, MSW, LSW Table of Contents OSAM-O-Gram....................................................................................................................................................................3 -

Commonly Used Drugs
Commonly Used Drugs Many drugs can alter a person’s thinking and judgment, and can lead to health risks, including addiction, drugged driving, infectious disease, and adverse effects on pregnancy. Information on commonly used drugs with the potential for misuse or addiction can be found here. For information about treatment options for substance use disorders, see NIDA’s Treatment pages. For drug use trends, see our Trends and Statistics page. For the most up-to-date slang terms, please see Slang Terms and Code Words: A Reference for Law Enforcement Personnel (DEA, PDF, 1MB). The following drugs are included in this resource: ➢ Alcohol ➢ Methamphetamine ➢ Ayahuasca ➢ Over-the-Counter Medicines--Dextromethorphan ➢ Central Nervous System Depressants (DXM) ➢ Cocaine ➢ Over-the-Counter Medicines--Loperamide ➢ DMT ➢ PCP ➢ GHB ➢ Prescription Opioids ➢ Hallucinogens ➢ Prescription Stimulants ➢ Heroin ➢ Psilocybin ➢ Inhalants ➢ Rohypnol® (Flunitrazepam) ➢ Ketamine ➢ Salvia ➢ Khat ➢ Steroids (Anabolic) ➢ Kratom ➢ Synthetic Cannabinoids ➢ LSD ➢ Synthetic Cathinones ("Bath Salts") ➢ Marijuana (Cannabis) ➢ Tobacco/Nicotine ➢ MDMA (Ecstasy/Molly) ➢ Mescaline (Peyote) **Drugs are classified into five distinct categories or schedules “depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential.” More information and the most up-to-date scheduling information can be found on the Drug Enforcement Administration’s website. June 2020 Alcohol People drink to socialize, celebrate, and relax. Alcohol often has a strong effect on people—and throughout history, people have struggled to understand and manage alcohol’s power. Why does alcohol cause people to act and feel differently? How much is too much? Why do some people become addicted while others do not? The National Institute on Alcohol Abuse and Alcoholism is researching the answers to these and many other questions about alcohol. -

Phencyclidine: an Update
Phencyclidine: An Update U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse and Mental Health Administration Phencyclidine: An Update Editor: Doris H. Clouet, Ph.D. Division of Preclinical Research National Institute on Drug Abuse and New York State Division of Substance Abuse Services NIDA Research Monograph 64 1986 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administratlon National Institute on Drug Abuse 5600 Fishers Lane Rockville, Maryland 20657 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National lnstitute on Drug Abuse and published by its Office of Science The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. SIDNEY COHEN, M.D. Temple University School of Medicine Los Angeles, California Philadelphia, Pennsylvania SYDNEY ARCHER, Ph.D. MARY L. JACOBSON Rensselaer Polytechnic lnstitute National Federation of Parents for Troy, New York Drug Free Youth RICHARD E. BELLEVILLE, Ph.D. Omaha, Nebraska NB Associates, Health Sciences Rockville, Maryland REESE T. JONES, M.D. KARST J. BESTEMAN Langley Porter Neuropsychiatric lnstitute Alcohol and Drug Problems Association San Francisco, California of North America Washington, D.C. DENISE KANDEL, Ph.D GILBERT J. BOTV N, Ph.D. College of Physicians and Surgeons of Cornell University Medical College Columbia University New York, New York New York, New York JOSEPH V.