BIRAC Annual Report (Inside Page)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2014 - 2015 Our Mission
ANNUAL REPORT 2014 - 2015 Our Mission By integrating the fields of medicine, science, engineering and technology into translational knowledge and making the resulting biomedical innovations accessible to public health, to improve the health of the most disadvantaged people in India and throughout the world. Our Vision As a networked organization linking many centers of excellence, THSTI is envisaged as a collective of scientists, engineers and physicians that will effectively enhance the quality of human life through integrating a culture of shared excellence in research, education and translational knowledge with the developing cohorts and studying the pathogenesis and the molecular mechanisms of disease to generate knowledge to complement the processes of designing interventions and technology development. CONTENTS 3 THE ORGANIZATION Society Governing Body Leadership From the Executive Director’s Desk 11 RESEARCH PROGRAMS Vaccine and Infectious Disease Research Centre Pediatric Biology Centre Centre for Biodesign and Diagnostics Policy Center for Biomedical Research Drug Discovery Research Centre Centre for Human Microbial Ecology Population Science Partnership Centre Clinical Development Services Agency 178 ACADEMIA 184 ADMINISTRATION Organization INTRAMURAL CENTRES Vaccine & Infectious Disease Research Centre (VIDRC) Pediatric Biology Centre (PBC) Centre for Biodesign & Diagnostics (CBD) Centre for Human Microbial Ecology (CHME) Policy Centre for Biomedical Research (PCBR) Drug Discovery Research Centre (DDRC) PARTNERSHIP CENTRE Population Science Partnership Centre (PSPC) EXTRAMURAL CENTRE Clinical Development Services Agency (CDSA) THSTI Society 1 2 3 4 5 6 7 8 9 10 11 12 1. Dr. G. Padmanaban 5. Dr. T.S. Rao 9. Dr. J. Gowrishankar Distinguished Professor, Nodal Officer, THSTI, Sr. Advisor, Director, IISc Bangalore Department of Biotechnology, Centre for DNA Fingerprinting President New Delhi & Diagnostics, Member Ex-officio Hyderabad 2. -

List of Officers Who Attended Courses at NCRB
List of officers who attened courses at NCRB Sr.No State/Organisation Name Rank YEAR 2000 SQL & RDBMS (INGRES) From 03/04/2000 to 20/04/2000 1 Andhra Pradesh Shri P. GOPALAKRISHNAMURTHY SI 2 Andhra Pradesh Shri P. MURALI KRISHNA INSPECTOR 3 Assam Shri AMULYA KUMAR DEKA SI 4 Delhi Shri SANDEEP KUMAR ASI 5 Gujarat Shri KALPESH DHIRAJLAL BHATT PWSI 6 Gujarat Shri SHRIDHAR NATVARRAO THAKARE PWSI 7 Jammu & Kashmir Shri TAHIR AHMED SI 8 Jammu & Kashmir Shri VIJAY KUMAR SI 9 Maharashtra Shri ABHIMAN SARKAR HEAD CONSTABLE 10 Maharashtra Shri MODAK YASHWANT MOHANIRAJ INSPECTOR 11 Mizoram Shri C. LALCHHUANKIMA ASI 12 Mizoram Shri F. RAMNGHAKLIANA ASI 13 Mizoram Shri MS. LALNUNTHARI HMAR ASI 14 Mizoram Shri R. ROTLUANGA ASI 15 Punjab Shri GURDEV SINGH INSPECTOR 16 Punjab Shri SUKHCHAIN SINGH SI 17 Tamil Nadu Shri JERALD ALEXANDER SI 18 Tamil Nadu Shri S. CHARLES SI 19 Tamil Nadu Shri SMT. C. KALAVATHEY INSPECTOR 20 Uttar Pradesh Shri INDU BHUSHAN NAUTIYAL SI 21 Uttar Pradesh Shri OM PRAKASH ARYA INSPECTOR 22 West Bengal Shri PARTHA PRATIM GUHA ASI 23 West Bengal Shri PURNA CHANDRA DUTTA ASI PC OPERATION & OFFICE AUTOMATION From 01/05/2000 to 12/05/2000 1 Andhra Pradesh Shri LALSAHEB BANDANAPUDI DY.SP 2 Andhra Pradesh Shri V. RUDRA KUMAR DY.SP 3 Border Security Force Shri ASHOK ARJUN PATIL DY.COMDT. 4 Border Security Force Shri DANIEL ADHIKARI DY.COMDT. 5 Border Security Force Shri DR. VINAYA BHARATI CMO 6 CISF Shri JISHNU PRASANNA MUKHERJEE ASST.COMDT. 7 CISF Shri K.K. SHARMA ASST.COMDT. -

Awardees of National Bioscience Award for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARD FOR CAREER DEVELOPMENT Awardees for the year 2016 1. Dr. Mukesh Jain, Associate Professor, School of Computational and Integrative Sciences, Jawaharlal Nehru University, New Delhi-110067 2. Dr. Samir K. Maji, Associate Professor, Indian Institute of Technology, Powai, Mumbai- 400076 3. Dr. Anindita Ukil, Assistant Professor, Calcutta University, Kolkata 4. Dr. Arnab Mukhopadhyay, Staff Scientist V, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi- 110067 5. Dr. Rohit Srivastava, Professor, Indian Institute of Technology, Bombay, Mumbai- 400076 6. Dr. Pinaki Talukdar, Associate Professor, Indian Institute of Science Education and Research, Dr. Homi Bhabha Road, Pashan, Pune- 7. Dr. Rajnish Kumar Chaturvedi, Senior Scientist, CSIR- Indian Institute of Toxicology Research, Lucknow-226001 8. Dr. Jackson James, Scientist E-II, Neuro Stem Cell Biology Lab, Neurobiology Division, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala- 695014 Awardees for the year 2015 1. Dr. Sanjeev Das, Staff Scientist-V, National Institute of Immunology, New Delhi 2. Dr. Ganesh Nagaraju, Assistant Professor, Department of Biotechnology, Indian Institute of Science, Bangalore- 5600012. 3. Dr. Suvendra Nath Bhattacharya, Principal Scientist, CSIR- Indian Institute of Chemical Biology, Kolkata- 700032 4. Dr. Thulasiram H V, Principal Scientist, CSIR-National Chemical Laboratory, Pune- 411008. 5. Dr. Pawan Gupta, Principal Scientist, Institute of microbial Technology, Chandigarh- 160036. 6. Dr. Souvik Maiti, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025. 7. Dr. Pravindra Kumar, Associate Professor, Department of Biotechnology, IIT, Roorkee- 247667. 8. Dr. Anurag Agrawal, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025 9. Dr. Gridhar Kumar Pandey, Professor, Department of Plant Molecular Biology, University of Delhi South Campus, New Delhi- 110067 10. -

National Bioscience Awards for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARDS FOR CAREER DEVELOPMENT Awardees for the year 2012 1. Dr. Kaustuv Sanyal, Associate Professor, Molecular Mycology Laboratory, Molecular Biology & Genetics Unit, Jawaharlal Nehru Centre for Advance Scientific Research, Jakkur P.O. Bangalore 560064 2. Dr Naval Kishore Vikram, Associate Professor, Department of Medicine, All India Institute of Medical Sciences (AIIMS), Ansari Nagar, New Delhi- 110029 3. Dr. Aditya Bhushan Pant, Senior Scientist & In-charge, In Vitro Toxicology Laboratory, Indian Institute of Toxicology Research, PO Box: 80, MG Marg, Lucknow 226001 (UP) India 4. Dr. Subrata Adak, Senior Scientist, Indian Institute of Chemical Biology; 4, Raja S.C. Mullick Road, Kolkata-700032 5. Dr. Durai Sundar, Assistant Professor, Dept of Biochemical Engineering & Biotechnology, Indian Institute of Technology (IIT) Delhi, Hauz Khas, New Delhi – 110016 6. Dr S Venkata Mohan, Senior Scientist, Bioengineering and Environmental Centre (BEEC) CSIR-Indian Institute of Chemical Technology, Hyderabad-500 607 7. Dr. Munia Ganguli, Scientist E-I, CSIR-Institute of Genomics & Integrative Biology, Mall Road,New Delhi 110 007 8. Dr. Asad U Khan, Associate Professor & Coordinator/Head of Biotechnology Department, A.M.U, Interdisciplinary Biotechnology Unit, A.M.U., Aligarh 202002 9. Dr. Sathees C. Raghavan, Assistant Professor, Department of Biochemistry, Indian Institute of Science, Bangalore 560 012 10. Dr. Vidita A. Vaidya, Associate Professor, Department of Biological Sciences, Tata Institute of Fundamental Research, 1, Homi Bhabha Road, Colaba, Mumbai - 400005 Awardees for the year 2011 1. Dr. M. M. Parida, Scientist-F, Joint Director, Division of Virology Defence R & D Establishment, DRDE, DRDO, Ministry of Defence, Jhansi Road, Gwalior- 474002 2. -
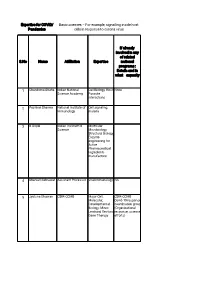
Basic Sciences – for Example, Signalling Inside Host Pandemics Cells in Response to Corona Virus
Expertise for COVID/ Basic sciences – For example, signalling inside host Pandemics cells in response to corona virus If already involved in any of related S.No Name Affiliation Expertise national programs : Details and in what capacity 1 Chandrima Shaha Indian National Cell Biology, Host None Science Academy Parasite interactions 2 Pushkar Sharma National Institute of Cell signaling, Immunology malaria 3 B Gopal Indian Institute of Molecular Science Microbiology, Structural Biology, Enzyme engineering for Active Pharmaceutical Ingredients manufacture 4 Sharvan Sehrawat Assistant Professor Viral immunology NA 5 Jyotsna Dhawan CSIR-CCMB Major-Cell, CSIR-CCMB Molecular, Covid-19 response Developmental coordination group Biology; Minor: (Organizational Lentiviral Vectors, response, science Gene Therapy efforts) 6 G. Baskaran The Institute of Theoretical Mathematical Physics. Sciences, Chennai Curious about 600 113 and biological IITMadras processes for decades. 7 Pradip Kumar Jamia Hamdard, NewMolecular NA Chakraborti Delhi 110062 Microbiology, Infectious disease, prokaryotic signalling 8 Nahid Ali CSIR Indian institute Parasitology, of chemical biology immunology 9 Raj Kumar Roy IISER Mohali Polymer and No Material Chemistry 10 Narinder K. Mehra All India Institute of COVID-19 and Medical Sciences immunity, Laboratory diagnosis 11 V. Ramanathan IIT(BHU) Varanasi Raman imaging Have been helping for disease the administrative diagnosis authorities of my city and my institute in making and distributing hand sanitizers. Till date have made around -

The Year Book 2019
THE YEAR BOOK 2019 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-2361 6094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2019 Indian Academy of Sciences Information in this Year Book is updated up to 22 February 2019. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Venugopal, M.S. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by Lotus Printers Pvt. Ltd., Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Memorandum of Association ................................................... 2 Role of the Academy ............................................................... 4 Statutes .................................................................................. 7 Council for the period 2019–2021 ............................................ 18 Office Bearers ......................................................................... 19 Former Presidents ................................................................... 20 Activities – a profile ................................................................. 21 Academy Document on Scientific Values ................................. 25 The Academy Trust ................................................................. 33 Section B: Professorships -

Immunology in India: an Emerging Story
cOMMentArY Immunology in India: an emerging story Kanury V S Rao Although immunological research is of only recent origin in India, it is nevertheless rapidly becoming an area of choice for young researchers in this country. he perennial appeal of immunology lies Tin the fact that it provides a window for almost every aspect of biological and biomedi- cal research. The plasticity of lymphocytes as http://www.nature.com/natureimmunology they switch among quiescence, active prolif- eration and various differentiation programs encapsulates the essence of both cell biology and the gene expression events that drive cell fate ‘decisions’. The structural and functional diversity of immunity-related receptors elicits questions in evolutionary biology, genetics, biochemistry, biophysics and structural biol- ogy. Although belated, this universe of excit- ing opportunities offered by immunological research is also rapidly being increasingly rec- Nature Publishing Group Group Nature Publishing 8 ognized by researchers in India. And with the present availability of resources and educated 200 manpower, the country is geared to mature © into an important contributor to this field. A historical perspective The history of medicine in India goes back to Susruta, the father of plastic surgery, is considered the originator of the rhinoplasty technique that remote antiquity, to a period between 4,000 has been practiced since 600 BCE. A detailed description of the procedure is provided in the Susruta and 900 BCE known as the ‘Vedic period’. Samhita. The procedure later spread from India to Persia and Egypt and, many centuries later, to Although early Vedic medicine represented an Europe and is still practiced today by some families in India and Nepal. -
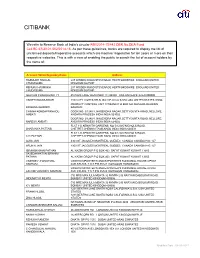
Details of Unclaimed Deposits / Inoperative Accounts
CITIBANK We refer to Reserve Bank of India’s circular RBI/2014-15/442 DBR.No.DEA Fund Cell.BC.67/30.01.002/2014-15. As per these guidelines, banks are required to display the list of unclaimed deposits/inoperative accounts which are inactive/ inoperative for ten years or more on their respective websites. This is with a view of enabling the public to search the list of account holders by the name of: Account Holder/Signatory Name Address HASMUKH HARILAL 207 WISDEN ROAD STEVENAGE HERTFORDSHIRE ENGLAND UNITED CHUDASAMA KINGDOM SG15NP RENUKA HASMUKH 207 WISDEN ROAD STEVENAGE HERTFORDSHIRE ENGLAND UNITED CHUDASAMA KINGDOM SG15NP GAUTAM CHAKRAVARTTY 45 PARK LANE WESTPORT CT.06880 USA UNITED STATES 000000 VAIDEHI MAJMUNDAR 1109 CITY LIGHTS DR ALISO VIEJO CA 92656 USA UNITED STATES 92656 PRODUCT CONTROL UNIT CITIBANK P O BOX 548 MANAMA BAHRAIN KRISHNA GUDDETI BAHRAIN CHINNA KONDAPPANAIDU DOOR NO: 3/1240/1, NAGENDRA NAGAR,SETTYGUNTA ROAD, NELLORE, AMBATI ANDHRA PRADESH INDIA INDIA 524002 DOOR NO: 3/1240/1, NAGENDRA NAGAR,SETTYGUNTA ROAD, NELLORE, NARESH AMBATI ANDHRA PRADESH INDIA INDIA 524002 FLAT 1-D,KENWITH GARDENS, NO 5/12,MC'NICHOLS ROAD, SHARANYA PATTABI CHETPET,CHENNAI TAMILNADU INDIA INDIA 600031 FLAT 1-D,KENWITH GARDENS, NO 5/12,MC'NICHOLS ROAD, C D PATTABI . CHETPET,CHENNAI TAMILNADU INDIA INDIA 600031 ALKA JAIN 4303 ST.JACQUES MONTREAL QUEBEC CANADA CANADA H4C 1J7 ARUN K JAIN 4303 ST.JACQUES MONTREAL QUEBEC CANADA CANADA H4C 1J7 IBRAHIM KHAN PATHAN AL KAZMI GROUP P O BOX 403 SAFAT KUWAIT KUWAIT 13005 SAJEDAKHATUN IBRAHIM PATHAN AL KAZMI -

The Year Book 2020
THE YEAR BOOK 2020 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-23616094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2020 Indian Academy of Sciences Information in this Year Book is updated up to 31 January 2020. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by The Print Point, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: -
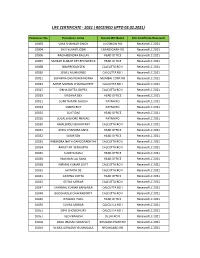
Life Certificate - 2021 ( Received Upto 02.02.2021)
LIFE CERTIFICATE - 2021 ( RECEIVED UPTO 02.02.2021) Pensioner No. Pensioner name Service RO Name Life Certificate Received 10003 UMA SHANKAR SINGH LUCKNOW RO Receivedstatus LC 2021 10004 SHIV KUMAR JOSHI CHANDIGARH RO Received LC 2021 10006 RAGHABENDRA BALLAV HEAD OFFICE Received LC 2021 10007 SANKAR KUMAR DEY BHOWMICK HEAD OFFICE Received LC 2021 10008 SIBAPROSAD SEN CALCUTTA RO II Received LC 2021 10010 JEWEL MUKHERJEE CALCUTTA RO I Received LC 2021 10011 SUPARNA DAS PURKAYASTHA MUMBAI CORP.RO Received LC 2021 10013 MIHIR MOHAN CHAKRAVORTY CALCUTTA RO I Received LC 2021 10017 SIKHA DUTTA GUPTA CALCUTTA RO II Received LC 2021 10019 KRISHNA DEY HEAD OFFICE Received LC 2021 10021 SUMITA RANI GHOSH PATNA RO Received LC 2021 10024 SABITA ROY PATNA RO Received LC 2021 10025 SUJIT DAS HEAD OFFICE Received LC 2021 10026 JUGAL KISHORE PRASAD PATNA RO Received LC 2021 10030 AMALENDU SEKHAR RAY CALCUTTA RO II Received LC 2021 10031 AKHIL CHANDRA SAHA HEAD OFFICE Received LC 2021 10032 SUBIR SEN HEAD OFFICE Received LC 2021 10033 RABINDRA NATH GANGOPADHYAY CALCUTTA RO II Received LC 2021 10034 RANJIT KR. SENGUPTA CALCUTTA RO II Received LC 2021 10035 SUMITA BASU HEAD OFFICE Received LC 2021 10036 MAKHAN LAL SAHA HEAD OFFICE Received LC 2021 10038 NIRMAL KUMAR DUTT CALCUTTA RO II Received LC 2021 10039 JAYANTA DE CALCUTTA RO II Received LC 2021 10041 APARNA DUTTA HEAD OFFICE Received LC 2021 10045 GITIKA SARKAR CALCUTTA RO II Received LC 2021 10047 SHYAMAL KUMAR BANERJEA CALCUTTA RO I Received LC 2021 10048 BUDDHADEB CHAKRABORTY CALCUTTA RO II Received -

2018-2019 As Against the Requirement of AS-15 Issued by ICAI
National Brain Research Centre Annual Report 2018-19 Mandate & Objectives .............................................................................7 From the Director’s Desk ........................................................................9 Scientific Reports ..................................................................................15 Dr. Anindya Ghosh Roy ........................................................................................................................17 Prof. Anirban Basu................................................................................................................................22 Dr. Arpan Banerjee ...............................................................................................................................27 Dr. Dipanjan Roy ..................................................................................................................................32 Prof. Ellora Sen ....................................................................................................................................42 Prof. Nandini C. Singh ..........................................................................................................................44 Prof. Neeraj Jain ...................................................................................................................................47 Prof. Pankaj Seth..................................................................................................................................52 Prof. Pravat -

The Year Book 2021
THE YEAR BOOK 2021 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-2361 6094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2021 Indian Academy of Sciences Information in this Year Book is updated up to 15 February 2021. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Mobile: 97310 01283) Printed by Ekakshara Printers, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: Fellowship