Tempering Large Tuna Prior to Thawing to Minimize Histamine Formation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

A Review of the Biology for Pacific Saury, Cololabis Saira in the North
North Pacific Fisheries Commission NPFC-2019-SSC PSSA05-WP13 (Rev. 1) A review of the biology for Pacific saury, Cololabis saira in the North Pacific Ocean Taiki Fuji1*, Satoshi Suyama2, Shin-ichiro Nakayama3, Midori Hashimoto1, Kazuhiro Oshima1 1National Research Institute of Far Seas Fisheries, Japan Fisheries Research and Education Agency 2Tohoku national Fisheries Research Institute, Japan Fisheries Research and Education Agency 3National Research Institute of Fisheries Science, Fisheries Research and Education Agency *Corresponding author’s email address: [email protected] Contents 1. Introduction…………………………………………………………………………………………2 2. Stock identity……………………………………………………………………………………….2 3. Early life history……………………………………………………………………………………2 3-1. Spawning ground………………………………………………………………………………2 3-2. Larval transportation……………………………………………………………………………3 3-3. Recruitment variability………………………………………………………………………….4 4. Feeding habits and predators…………………………………………………………………………4 5. Growth………………………………………………………………………………………………..5 6. Maturation…………………………………………………………………………………………….5 6-1. Spawning pattern, fecundity and spawning duration…………………………………………….5 6-2. Seasonal change of maturity size………………………………...................................................6 6-3. Maturation schedule for each seasonal cohort considering growth and maturation size…………6 6-4. Maturation and environmental factors……………………………………………………………7 6-5. Percentage of matured fish………………………………………………………………………..7 7. Distribution and migration…………………………………………………………………………….7 8. Natural mortality………………………………………………………………………………………9 -
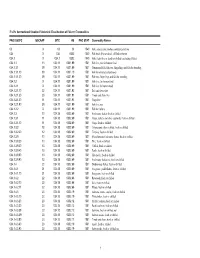
FAO's International Standard Statistical Classification of Fishery Commodities
FAO's International Standard Statistical Classification of Fishery Commodities FAO ISSCFC ISSCAAP SITC HS FAO STAT Commodity Names 03 X 03 03 1540 Fish, crustaceans, molluscs and preparations 034 X 034 0302 1540 Fish fresh (live or dead), chilled or frozen 034.1 X 034.1 0302 1540 Fish, fresh (live or dead) or chilled (excluding fillets) 034.1.1 13 034.11 0301.99 1501 Fish live, not for human food 034.1.1.1 39 034.11 0301.99 1501 Ornamental fish, fish ova, fingerlings and fish for breeding 034.1.1.1.10 39 034.11 0301.10 1501 Fish for ornamental purposes 034.1.1.1.20 39 034.11 0301.99 1501 Fish ova, fingerlings and fish for breeding 034.1.2 X 034.110301.99 1501 Fish live, for human food 034.1.2.1 X 034.110301.99 1501 Fish live for human food 034.1.2.1.10 22 034.11 0301.92 1501 Eels and elvers live 034.1.2.1.20 23 034.11 0301.91 1501 Trouts and chars live 034.1.2.1.30 11 034.11 0301.93 1501 Carps live 034.1.2.1.90 39 034.11 0301.99 1501 Fish live, nei 034.1.2.2 X 034.110301.99 1501 Fish for culture 034.1.3 10 034.18 0302.69 1501 Freshwater fishes, fresh or chilled 034.1.3.1 11 034.18 0302.69 1501 Carps, barbels and other cyprinids, fresh or chilled 034.1.3.1.10 11 034.18 0302.69 1501 Carps, fresh or chilled 034.1.3.2 12 034.18 0302.69 1501 Tilapias and other cichlids, fresh or chilled 034.1.3.2.20 12 034.18 0302.69 1501 Tilapias, fresh or chilled 034.1.3.9 10 034.18 0302.69 1501 Miscellaneous freshwater fishes, fresh or chilled 034.1.3.9.20 13 034.18 0302.69 1501 Pike, fresh or chilled 034.1.3.9.30 13 034.18 0302.69 1501 Catfish, fresh or -

Ocean Leader Co. Ltd. a Premium Seafood Exporter and Processor Key Milestones
Ocean Leader Co. Ltd. A Premium Seafood Exporter and Processor Key Milestones Founded in 1975, Ocean Leader Relocate our business focus on Ocean Leader Co. Ltd. became one of started as a loyal partner with the pacific fishery and became the biggest exporter of Mahi-mahi with multi Tai wanese seafood one of the leading exporter in other expertise in Wahoo, Mackerel, processer helping them selling Tai wan, exporting more than Blue Shark, Sword Fish, Sail Fish, King seafood to US 10,000 tons of fishery annually Fish, Tilapia...etc. 1975 1977 1990 1991-2018 Focused on shrimp business as Tai wan held Awarded ”Taiwan Excellent Exporter” for 30 the leading position in years consecutively global shrimp market Certified by HACCP, SQF, SGS and BRC, Ocean Leader Co. Ltd., demonstrated the pursuit in products sanitation and safety 2 Global Footprint Canada Europe USA Bahama UK Jamaica Dominica Puerto Rico Trinidad Mexico Ecuador 3 Products Overview Mahi Mahi Mackerel FRESH IQF Sword Fish Black Marlin King Fish Wahoo SEAFOOD Blue Shark Sail Fish CERTIFIED Golden Yellowfin Tuna Pompano Pacific Saury Oil Fish Mackerel Pike 4 Mahi Mahi Coryphaena Hippurus Product Specification: • Whole gutted • Fillet • Loin • Portion • Steak *All pictures are for illustration purpose only 5 Blue Shark Priunace Glauca Product Specification: • H+G (Headed & Gutted) • Fillet • Portion • Steak • Cube *All pictures are for illustration purpose only 6 Mackerel Scomber Japonicus Product Specification: • Whole round • Fillet *All pictures are for illustration purpose only 7 -

Opportunities for Sustainable Fisheries in Japan
OPPORTUNITIES FOR SUSTAINABLE FISHERIES IN JAPAN O2 REPORT: OPPORTUNITIES FOR SUSTAINABLE FISHERIES IN JAPAN JANUARY 2016 THIS REPORT OFFERS PRACTICAL RECOMMENDATIONS TO HELP RESTORE FISHERIES AND COASTAL FISHING COMMUNITIES ACROSS THE JAPANESE ARCHIPELAGO © Ana Chang 2 CONTENT Introduction/Summary 4 State of Japanese Fisheries 5 The Japanese Seafood Supply Chain 8 Seafood Supply Chain - Upstream 8 Seafood Supply Chain - Downstream 9 Seafood Imports/Exports 11 Species in Focus: Tuna Supply Chain 12 Policy/Management 14 Sustainable Seafood in Japan 17 Survey of Japanese Consumers 17 Survey of Japanese Fishermen/Managers 18 Recommendations 19 References 21 Addendum: Rapid Assessments of Eleven Japanese Fisheries 3 Introduction/Summary If you want to witness a display of marine abundance and diversity unrivaled nearly anywhere on planet earth, don’t go to the Coral Triangle. Instead, head straight to the heart of Tokyo, grab your rubber boots and take a stroll through the cavernous Tsukiji fish market. From wild Kamchatka sockeye salmon to giant tuna from the Mediterranean to Maine lobster, Tsukiji sells it all in the largest seafood market in the world. The freshest and highest quality seafood in Tsukiji still comes from waters sur- rounding the Japanese archipelago, which hold some of the most productive fishing grounds on the planet. But domestic fisheries have been in decline for decades, due to overfishing, degraded ecosystems, and negative socio-economic factors. For the average Japanese consumer, this decline has caused higher prices at the market and increasing difficulties in enjoying traditional “washoku” food items. “Unagi” (eel), for example, went from a peak commercial catch of 232 metric tons in 1963 to a measly 5 tons by 2011.1 Meanwhile, the price quadrupled in the last decade alone. -

Recycled Fish Sculpture (.PDF)
Recycled Fish Sculpture Name:__________ Fish: are a paraphyletic group of organisms that consist of all gill-bearing aquatic vertebrate animals that lack limbs with digits. At 32,000 species, fish exhibit greater species diversity than any other group of vertebrates. Sculpture: is three-dimensional artwork created by shaping or combining hard materials—typically stone such as marble—or metal, glass, or wood. Softer ("plastic") materials can also be used, such as clay, textiles, plastics, polymers and softer metals. They may be assembled such as by welding or gluing or by firing, molded or cast. Researched Photo Source: Alaskan Rainbow STEP ONE: CHOOSE one fish from the attached Fish Names list. Trout STEP TWO: RESEARCH on-line and complete the attached K/U Fish Research Sheet. STEP THREE: DRAW 3 conceptual sketches with colour pencil crayons of possible visual images that represent your researched fish. STEP FOUR: Once your fish designs are approved by the teacher, DRAW a representational outline of your fish on the 18 x24 and then add VALUE and COLOUR . CONSIDER: Individual shapes and forms for the various parts you will cut out of recycled pop aluminum cans (such as individual scales, gills, fins etc.) STEP FIVE: CUT OUT using scissors the various individual sections of your chosen fish from recycled pop aluminum cans. OVERLAY them on top of your 18 x 24 Representational Outline 18 x 24 Drawing representational drawing to judge the shape and size of each piece. STEP SIX: Once you have cut out all your shapes and forms, GLUE the various pieces together with a glue gun. -

Growth and Maturation of Pacific Saury Cololabis Saira Under Laboratory Conditions
Title Growth and maturation of Pacific saury Cololabis saira under laboratory conditions Nakaya, Mitsuhiro; Morioka, Taizo; Fukunaga, Kyouhei; Murakami, Naoto; Ichikawa, Takashi; Sekiya, Sachio; Author(s) Suyama, Satoshi Fisheries Science, 76(1), 45-53 Citation https://doi.org/10.1007/s12562-009-0179-9 Issue Date 2010-01 Doc URL http://hdl.handle.net/2115/74498 © 2016 公益社団法人日本水産学会; The final publication is available at www.springerlink.com; © 2016 The Rights Japanese Society of Fisheries Science Type article (author version) File Information 11_FS76 Nakaya et al.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 Growth and maturation of Pacific saury Cololabis saira under laboratory 2 conditions 3 Mitsuhiro Nakaya · Taizo Morioka · Kyouhei Fukunaga · Naoto Murakami · Takashi Ichikawa 4 · Sachio Sekiya · Satoshi Suyama 5 6 M. Nakaya (✉) · T. Morioka · K. Fukunaga · N. Murakami · T. Ichikawa 7 Hokkaido National Fisheries Research Institute, Fisheries Research Agency, Akkeshi, Akkeshi, Hokkaido 8 088-1108, Japan 9 S. Sekiya 10 Management Section of National Center for Stock Enhancement, Fisheries Research Agency, Yokohama, 11 Kanagawa 220-6115, Japan 12 S. Suyama 13 Hachinohe Branch, Tohoku National Fisheries Research Institute, Hachinohe, Aomori 031-0841, Japan 14 Present address: 15 M. Nakaya 16 Kakikin Corporation, Akkeshi, Hokkaido 088-1111, Japan 17 e-mail: [email protected] 18 Tel & Fax: +81-135-52-5277 19 1 20 Abstract This study details the growth and maturation processes of Pacific saury Cololabis saira from 21 eggs to first spawning under laboratory conditions. They were reared in 20ºC, and fed almost to 22 satiation every day. There was no significant difference in the knob length (KnL) between males and 23 females and therefore data were combined in the following Gompertz growth formula KnLt = 277.1 exp 24 (-exp (-0.015 (t - 83.8))). -

Fish in Disguise: Seafood Fraud in Korea
Fish in disguise: Seafood fraud in Korea A briefing by the Environmental Justice Foundation 1 Executive summary Between January and December 2018, the Environmental Justice Foundation (EJF) used DNA testing to determine levels of seafood fraud in the Republic of Korea. The results showed that over a third of samples tested were mislabelled. This mislabelling defrauds consumers, risks public health, harms the marine environment and can be associated with serious human rights abuses across the world. These findings demonstrate the urgent need for greater transparency and traceability in Korean seafood, including imported products. Key findings: • Over a third of seafood samples (34.8%, 105 of 302 samples) genetically analysed were mislabelled. • Samples labelled Fleshy Prawn, Fenneropenaeus chinensis (100%), Japanese Eel, Anguilla japonica (67.7%), Mottled Skate, Raja pulchra (53.3%) and Common Octopus, Octopus vulgaris (52.9%) had the highest rates of mislabelling. • Not a single sample labelled Fleshly Prawn was the correct species. • Mislabelling was higher in restaurants, fish markets and online than in general markets or superstores. • By processed types, sushi (53.9%), fresh fish (38.9%) and sashimi (33.6%) were the most likely to be mislabelled. • The seafood fraud identified by this research has direct negative impacts for consumers. It is clear that for some species sampled consumers were likely to be paying more than they should. For example, more than half of the eel and skate samples that were labelled domestic were actually found to be imported, which can cost only half of the price of domestic products. Swordfish mislabelled as Bluefin Tuna can be sold for four to five times as much. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Why Do Some Pelagic Fishes Have Wide Fluctuations in Their Numbers? ---Biological Basis of Fluctuation from the Viewpoint of Evolutionary Ecology
WHY DO SOME PELAGIC FISHES HAVE WIDE FLUCTUATIONS IN THEIR NUMBERS? ---BIOLOGICAL BASIS OF FLUCTUATION FROM THE VIEWPOINT OF EVOLUTIONARY ECOLOGY--- by Tsuyoshi Kawasaki Faculty of Agriculture Tohoku University 1–1 Amamiya-cho Tsutsumi-dori Sendai-shi 980, Japan Resumen Los patrones de fluctuación en número de individuos varían de una especie (sub-población) a otra, los que han sido seleccionados a través del pro- ceso de evolución. En el caso de teleósteos marinos se presentan tres tipos extremos de patrones de fluctuación, IA, IB y II, representados respectiva- mente por saury y amodites, arenque y sardina, atunes y peces planos. Una relación entre estos tres tipos puede ser expresada por un triángulo con dimensiones de longevidad, fecundidad y tasa de crecimiento. El tipo IA, muestra cambios espaciados de breve tiempo, es una especie de vida corta, tiene una fecundidad baja, y el producto de k (parámetro de crecimiento de la ecuación de Bertalanffy) y T (tiempo generación) es bajo, lo que hace que la tasa instantánea de incremento natural de la población (r) sea alta, mientras que el tipo IB, se caracteriza por mostrar variaciones fenomenales de largo tiempo, son especies de vida larga, menos fecundas y el producto de kT es alto, y además acumulan una gran cantidad de peces cuando se presenta una sucesión de clases anuales fuertes a pesar de una r baja. El tipo II tiene una biomasa estable, son de vida larga, son más fecundos y el producto de kT es bajo así como r. El patrón de fluctuación específico para esta especie depende mucho de las condiciones ambientales bióticas y abióticas en que se desarrolla la especie. -

Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Cruise Report of the Second Phase of the Japanese Whale Research
SC/60O7 SC/60/O7 Cruise report of the Second phase of the Japanese Whale Research Program under Special Permit in the western North Pacific (JARPN II) in 2007 – Coastal component off Kushiro TOSHIYA KISHIRO1, HIDEHIRO KATO2, HIDEYOSHI YOSHIDA1, TOMIO MIYASHITA1, TOSHIHIDE IWASAKI1, YU KANAJI1, TAMEO RYONO3, SHIGEO TABATA3, YUKA MORITA3, RYOSUKE OKAMOTO2, HIKARI MAEDA2, SYOKA NAGATUKA2, NATUKO OGAWA2, KAZUYOSHI NAKAI3, TADASHI EBISUI3, AKIRA MATUMOTO2, AKIHIRO GOKITA2, HIROSHI KIWADA3 AND SHIGEYUKI KAWAHARA1 Contact e-mail: [email protected] 1 National Research Institute of Far Seas Fisheries, 2-12-4, Fukuura, Kanazawa, Yokohama Kanazawa 236-8648, Japan 2Tokyo University of Marine Science and Technology, 1-5-7, Konan, Minato-ku, Tokyo, 108-0075 Japan 3 The Institute of Cetacean Research, 4-5, Toyomi-cho, Chuo-ku, Tokyo, 104-0055, Japan. ABSTRACT The fifth survey of the JARPN II coastal component off Kushiro, northeast Japan (northern part of the sub-area 7) was conducted from 10 September to 31 October 2007, using four small-type whaling catcher boats and one echo sounder-trawler and dedicated sighting survey vessel. The sampling was conducted in the coastal waters within the 50 nautical miles from the Kushiro port, and all whales sampled were landed on the land station in the Kushiro port for biological examination. During the survey, a total of 6,827.7 n. miles (637.6 hours) was searched for whale sampling, 98 schools/ 99 individuals of common minke whales were sighted and 50 whales were sampled. Average body length of sampled whales was 6.45m (SD=1.10, n=33) for males and 5.49m (SD=0.81, n=17) for females, respectively.