Why Do Some Pelagic Fishes Have Wide Fluctuations in Their Numbers? ---Biological Basis of Fluctuation from the Viewpoint of Evolutionary Ecology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Some Rare and Insufficiently Studied Snailfish (Liparidae
International Scholarly Research Network ISRN Zoology Volume 2011, Article ID 341640, 12 pages doi:10.5402/2011/341640 Review Article Some Rare and Insufficiently Studied Snailfish (Liparidae, Scorpaeniformes, Pisces) in the Pacific Waters off the Northern Kuril Islands and Southeastern Kamchatka, Russia A. M. Orlov1 andA.M.Tokranov2 1 Laboratory of Atlantic Basin, Department of International Fisheries Cooperation, Russian Federal Research Institute of Fisheries and Oceanography (VNIRO), 17 V. Krasnoselskaya, Moscow 107140, Russia 2 Kamchatka Branch of Pacific Institute of Geography, Far East Branch of Russian Academy of Sciences, 6 Partizanskaya, Petropavlovsk-Kamchatsky 683000, Russia CorrespondenceshouldbeaddressedtoA.M.Orlov,[email protected] Received 19 January 2011; Accepted 13 March 2011 Academic Editors: D. Park and M. Mooring Copyright © 2011 A. M. Orlov and A. M. Tokranov. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Spatial and vertical distributions, size-weight compositions, age, and diets of 10 rare or poorly known snailfish (Liparidae) from the Pacific off the southeastern Kamchatka and the northern Kuril Islands are described. The species include blacktip snailfish Careproctus zachirus, Alaska snailfish C. colletti, blacktail snailfish C. melanurus, proboscis snailfish C. simus, falcate snailfish C. cypselurus, big-disc snailfish Squaloliparis dentatus, longtip snailfish Elassodiscus obscurus, slender snailfish Paraliparis grandis, gloved snailfish Palmoliparis beckeri, and stout snailfish Allocareproctus jordani. These species inhabit a wide range of depths. Careproctus melanurus, C. cypselurus, E. obscurus, P. grandis, and C. colletti are the deepest; C. simus and S. dentatus occur mostly between 300 and 600 m; the three other species seldom occur at depths of 150–200 m. -

Nursery Origin and Population Connectivity of Swordfish Xiphias Gladius in the North Pacific Ocean
Received: 15 January 2021 Accepted: 9 March 2021 DOI: 10.1111/jfb.14723 REGULAR PAPER FISH Nursery origin and population connectivity of swordfish Xiphias gladius in the North Pacific Ocean R. J. David Wells1,2 | Veronica A. Quesnell1 | Robert L. Humphreys Jr3 | Heidi Dewar4 | Jay R. Rooker1,2 | Jaime Alvarado Bremer1,2 | Owyn E. Snodgrass4 1Department of Marine Biology, Texas A&M University at Galveston, Galveston, Texas Abstract 2Department of Ecology & Conservation Element:Ca ratios in the otolith cores of young-of-the-year (YOY) swordfish, Xiphias Biology, Texas A&M University, College gladius, were used as natural tracers to predict the nursery origin of subadult and adult Station, Texas 3Retired, Pacific Islands Fisheries Science swordfish from three foraging grounds in the North Pacific Ocean (NPO). First, the Center, National Marine Fisheries Service, chemistry of otolith cores (proxy for nursery origin) was used to develop nursery- Honolulu, Hawaii specific elemental signatures in YOY swordfish. Sagittal otoliths of YOY swordfish were 4Southwest Fisheries Science Center, National Marine Fisheries Service, La Jolla, California collected from four regional nurseries in the NPO between 2000 and 2005: (1) Central Equatorial North Pacific Ocean (CENPO), (2) Central North Pacific Ocean (CNPO), Correspondence R. J. David Wells, Texas A&M University at (3) Eastern Equatorial North Pacific Ocean (EENPO) and (4) Western North Pacific Galveston, Department of Marine Biology, Ocean (WNPO). Calcium (43Ca), magnesium (24Mg), strontium (88Sr) and barium (138Ba) 1001 Texas Clipper Rd. Galveston, TX 77554, USA. were quantified in the otolith cores of YOY swordfish using laser ablation inductively Email: [email protected] coupled plasma mass spectrometry. -
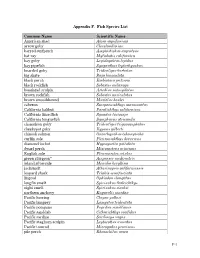
Appendix E: Fish Species List
Appendix F. Fish Species List Common Name Scientific Name American shad Alosa sapidissima arrow goby Clevelandia ios barred surfperch Amphistichus argenteus bat ray Myliobatis californica bay goby Lepidogobius lepidus bay pipefish Syngnathus leptorhynchus bearded goby Tridentiger barbatus big skate Raja binoculata black perch Embiotoca jacksoni black rockfish Sebastes melanops bonehead sculpin Artedius notospilotus brown rockfish Sebastes auriculatus brown smoothhound Mustelus henlei cabezon Scorpaenichthys marmoratus California halibut Paralichthys californicus California lizardfish Synodus lucioceps California tonguefish Symphurus atricauda chameleon goby Tridentiger trigonocephalus cheekspot goby Ilypnus gilberti chinook salmon Oncorhynchus tshawytscha curlfin sole Pleuronichthys decurrens diamond turbot Hypsopsetta guttulata dwarf perch Micrometrus minimus English sole Pleuronectes vetulus green sturgeon* Acipenser medirostris inland silverside Menidia beryllina jacksmelt Atherinopsis californiensis leopard shark Triakis semifasciata lingcod Ophiodon elongatus longfin smelt Spirinchus thaleichthys night smelt Spirinchus starksi northern anchovy Engraulis mordax Pacific herring Clupea pallasi Pacific lamprey Lampetra tridentata Pacific pompano Peprilus simillimus Pacific sanddab Citharichthys sordidus Pacific sardine Sardinops sagax Pacific staghorn sculpin Leptocottus armatus Pacific tomcod Microgadus proximus pile perch Rhacochilus vacca F-1 plainfin midshipman Porichthys notatus rainwater killifish Lucania parva river lamprey Lampetra -

Title First Records of the Snailfish Careproctus Lycopersicus (Cottoidei
First Records of the Snailfish Careproctus lycopersicus Title (Cottoidei: Liparidae) from the Western North Pacific Author(s) Kai, Yoshiaki; Matsuzaki, Koji; Mori, Toshiaki Citation Species Diversity (2019), 24(2): 115-118 Issue Date 2019-07-25 URL http://hdl.handle.net/2433/253532 © 2019 The Japanese Society of Systematic Zoology; 許諾条 Right 件に基づいて掲載しています。 Type Journal Article Textversion publisher Kyoto University Species Diversity 24: 115–118 Published online 25 July 2019 DOI: 10.12782/specdiv.24.115 First Records of the Snailfish Careproctus lycopersicus (Cottoidei: Liparidae) from the Western North Pacific Yoshiaki Kai1,3, Koji Matsuzaki2, and Toshiaki Mori2 1 Maizuru Fisheries Research Station, Field Science Education and Research Center, Kyoto University, Nagahama, Maizuru, Kyoto 625-0086, Japan E-mail: [email protected] 2 Marine Science Museum, Fukushima (Aquamarine Fukushima), Onahama, Iwaki, Fukushima 971-8101, Japan 3 Corresponding author (Received 8 March 2019; Accepted 14 May 2019) Four specimens (168.6–204.4 mm standard length) of Careproctus lycopersicus Orr, 2012, previously recorded from the Bering Sea and eastern Aleutian Islands, were collected from the southern Sea of Okhotsk (the Nemuro Strait, eastern Hokkaido, Japan). These specimens represent the first records of the species from the western North Pacific. A detailed description is provided for the specimens, including the intraspecific variations. The new standard Japanese name “Tomato- kon’nyaku-uo” is proposed for the species. Key Words: Teleostei, Actinopterygii, Sea of Okhotsk, Japan, distribution. Introduction Materials and Methods Snailfishes of the family Liparidae Scopoli, 1777 compose Counts, measurements, and descriptive terminology fol- a large and diverse group in the suborder Cottoidei, hav- low Orr and Maslenikov (2007). -

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Management Plan for the Rougheye/Blackspotted Rockfish Complex (Sebastes Aleutianus and S
DRAFT SPECIES AT RISK ACT MANAGESPECIESMENT PLAN AT RISK SERIES ACT MANAGEMENT PLAN SERIES MANAGEMENT PLAN FOR THE ROUGHEYE/BLACKSPOTTEDMANAGEMENT PLAN FOR THE ROCKFISH ROUGHEY E COMPLEXROCKFISHROUGHEYE (SEBASTES ALEUTIANUSROCKFISH COMPLEX AND S. MELANOSTICTUS(SEBASTES ALEUTIANUS) AND LONGSPINE AND S. THORNYHEADMELANOSTICTUS (SEBASTOLOBUS) AND LONGSPINE ALTIVELIS THORNYHE) IN AD CANADA(SEBAST OLOBUS ALTIVELIS)INCANADA SEBASTES ALEUTIANUS; SEBASTES MELANOSTICTUS SEBASTES ASEBASTOLOBUSLEUTIANUS; SEBASTES ALTIVELIS MEL ANOSTICTUS SEBASTOLOBUS ALTIVELIS S. aleutianus S. melanostictus 2012 Photo Credit: DFO Sebastolobus altivelis 2012 About the Species at Risk Act Management Plan Series What is the Species at Risk Act (SARA)? SARA is the Act developed by the federal government as a key contribution to the common national effort to protect and conserve species at risk in Canada. SARA came into force in 2003, and one of its purposes is “to manage species of special concern to prevent them from becoming endangered or threatened.” What is a species of special concern? Under SARA, a species of special concern is a wildlife species that could become threatened or endangered because of a combination of biological characteristics and identified threats. Species of special concern are included in the SARA List of Wildlife Species at Risk. What is a management plan? Under SARA, a management plan is an action-oriented planning document that identifies the conservation activities and land use measures needed to ensure, at a minimum, that a species of special concern does not become threatened or endangered. For many species, the ultimate aim of the management plan will be to alleviate human threats and remove the species from the List of Wildlife Species at Risk. -

Zootaxa, Snailfish Genus Allocareproctus (Teleostei
Zootaxa 1173: 1–37 (2006) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1173 Copyright © 2006 Magnolia Press ISSN 1175-5334 (online edition) Revision of the snailfish genus Allocareproctus Pitruk & Fedorov (Teleostei: Liparidae), with descriptions of four new species from the Aleutian Islands JAMES WILDER ORR1 & MORGAN SCOTT BUSBY2 National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Alaska Fisheries Sci- ence Center, Resource Assessment and Conservation Engineering Division, 7600 Sand Point Way NE, Build- ing 4, Seattle, WA 98115, U.S.A; E-mail: [email protected]; [email protected] Table of contents Abstract ............................................................................................................................................. 1 Introduction ....................................................................................................................................... 2 Method and materials ........................................................................................................................ 3 Systematic accounts .......................................................................................................................... 4 Allocareproctus Pitruk & Fedorov 1993 .................................................................................... 4 Key to species of Allocareproctus ............................................................................................13 Allocareproctus jordani (Burke 1930) .....................................................................................14 -

A Review of the Biology for Pacific Saury, Cololabis Saira in the North
North Pacific Fisheries Commission NPFC-2019-SSC PSSA05-WP13 (Rev. 1) A review of the biology for Pacific saury, Cololabis saira in the North Pacific Ocean Taiki Fuji1*, Satoshi Suyama2, Shin-ichiro Nakayama3, Midori Hashimoto1, Kazuhiro Oshima1 1National Research Institute of Far Seas Fisheries, Japan Fisheries Research and Education Agency 2Tohoku national Fisheries Research Institute, Japan Fisheries Research and Education Agency 3National Research Institute of Fisheries Science, Fisheries Research and Education Agency *Corresponding author’s email address: [email protected] Contents 1. Introduction…………………………………………………………………………………………2 2. Stock identity……………………………………………………………………………………….2 3. Early life history……………………………………………………………………………………2 3-1. Spawning ground………………………………………………………………………………2 3-2. Larval transportation……………………………………………………………………………3 3-3. Recruitment variability………………………………………………………………………….4 4. Feeding habits and predators…………………………………………………………………………4 5. Growth………………………………………………………………………………………………..5 6. Maturation…………………………………………………………………………………………….5 6-1. Spawning pattern, fecundity and spawning duration…………………………………………….5 6-2. Seasonal change of maturity size………………………………...................................................6 6-3. Maturation schedule for each seasonal cohort considering growth and maturation size…………6 6-4. Maturation and environmental factors……………………………………………………………7 6-5. Percentage of matured fish………………………………………………………………………..7 7. Distribution and migration…………………………………………………………………………….7 8. Natural mortality………………………………………………………………………………………9 -

Periodic Status Review for the Steller Sea Lion
STATE OF WASHINGTON January 2015 Periodic Status Review for the Steller Sea Lion Gary J. Wiles The Washington Department of Fish and Wildlife maintains a list of endangered, threatened, and sensitive species (Washington Administrative Codes 232-12-014 and 232-12-011, Appendix E). In 1990, the Washington Wildlife Commission adopted listing procedures developed by a group of citizens, interest groups, and state and federal agencies (Washington Administrative Code 232-12-297, Appendix A). The procedures include how species listings will be initiated, criteria for listing and delisting, a requirement for public review, the development of recovery or management plans, and the periodic review of listed species. The Washington Department of Fish and Wildlife is directed to conduct reviews of each endangered, threatened, or sensitive wildlife species at least every five years after the date of its listing. The reviews are designed to include an update of the species status report to determine whether the status of the species warrants its current listing status or deserves reclassification. The agency notifies the general public and specific parties who have expressed their interest to the Department of the periodic status review at least one year prior to the five-year period so that they may submit new scientific data to be included in the review. The agency notifies the public of its recommendation at least 30 days prior to presenting the findings to the Fish and Wildlife Commission. In addition, if the agency determines that new information suggests that the classification of a species should be changed from its present state, the agency prepares documents to determine the environmental consequences of adopting the recommendations pursuant to requirements of the State Environmental Policy Act. -
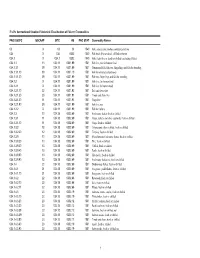
FAO's International Standard Statistical Classification of Fishery Commodities
FAO's International Standard Statistical Classification of Fishery Commodities FAO ISSCFC ISSCAAP SITC HS FAO STAT Commodity Names 03 X 03 03 1540 Fish, crustaceans, molluscs and preparations 034 X 034 0302 1540 Fish fresh (live or dead), chilled or frozen 034.1 X 034.1 0302 1540 Fish, fresh (live or dead) or chilled (excluding fillets) 034.1.1 13 034.11 0301.99 1501 Fish live, not for human food 034.1.1.1 39 034.11 0301.99 1501 Ornamental fish, fish ova, fingerlings and fish for breeding 034.1.1.1.10 39 034.11 0301.10 1501 Fish for ornamental purposes 034.1.1.1.20 39 034.11 0301.99 1501 Fish ova, fingerlings and fish for breeding 034.1.2 X 034.110301.99 1501 Fish live, for human food 034.1.2.1 X 034.110301.99 1501 Fish live for human food 034.1.2.1.10 22 034.11 0301.92 1501 Eels and elvers live 034.1.2.1.20 23 034.11 0301.91 1501 Trouts and chars live 034.1.2.1.30 11 034.11 0301.93 1501 Carps live 034.1.2.1.90 39 034.11 0301.99 1501 Fish live, nei 034.1.2.2 X 034.110301.99 1501 Fish for culture 034.1.3 10 034.18 0302.69 1501 Freshwater fishes, fresh or chilled 034.1.3.1 11 034.18 0302.69 1501 Carps, barbels and other cyprinids, fresh or chilled 034.1.3.1.10 11 034.18 0302.69 1501 Carps, fresh or chilled 034.1.3.2 12 034.18 0302.69 1501 Tilapias and other cichlids, fresh or chilled 034.1.3.2.20 12 034.18 0302.69 1501 Tilapias, fresh or chilled 034.1.3.9 10 034.18 0302.69 1501 Miscellaneous freshwater fishes, fresh or chilled 034.1.3.9.20 13 034.18 0302.69 1501 Pike, fresh or chilled 034.1.3.9.30 13 034.18 0302.69 1501 Catfish, fresh or -

The Morphology and Sculpture of Ossicles in the Cyclopteridae and Liparidae (Teleostei) of the Baltic Sea
Estonian Journal of Earth Sciences, 2010, 59, 4, 263–276 doi: 10.3176/earth.2010.4.03 The morphology and sculpture of ossicles in the Cyclopteridae and Liparidae (Teleostei) of the Baltic Sea Tiiu Märssa, Janek Leesb, Mark V. H. Wilsonc, Toomas Saatb and Heli Špilevb a Institute of Geology at Tallinn University of Technology, Ehitajate tee 5, 19086 Tallinn, Estonia; [email protected] b Estonian Marine Institute, University of Tartu, Mäealuse Street 14, 12618 Tallinn, Estonia; [email protected], [email protected], [email protected] c Department of Biological Sciences and Laboratory for Vertebrate Paleontology, University of Alberta, Edmonton, Alberta T6G 2E9 Canada; [email protected] Received 31 August 2009, accepted 28 June 2010 Abstract. Small to very small bones (ossicles) in one species each of the families Cyclopteridae and Liparidae (Cottiformes) of the Baltic Sea are described and for the first time illustrated with SEM images. These ossicles, mostly of dermal origin, include dermal platelets, scutes, tubercles, prickles and sensory line segments. This work was undertaken to reveal characteristics of the morphology, sculpture and ultrasculpture of these small ossicles that could be useful as additional features in taxonomy and systematics, in a manner similar to their use in fossil material. The scutes and tubercles of the cyclopterid Cyclopterus lumpus Linnaeus are built of small denticles, each having its own cavity viscerally. The thumbtack prickles of the liparid Liparis liparis (Linnaeus) have a tiny spinule on a porous basal plate; the small size of the prickles seems to be related to their occurrence in the exceptionally thin skin, to an adaptation for minimizing weight and/or metabolic cost and possibly to their evolution from isolated ctenii no longer attached to the scale plates of ctenoid scales. -

Ocean Leader Co. Ltd. a Premium Seafood Exporter and Processor Key Milestones
Ocean Leader Co. Ltd. A Premium Seafood Exporter and Processor Key Milestones Founded in 1975, Ocean Leader Relocate our business focus on Ocean Leader Co. Ltd. became one of started as a loyal partner with the pacific fishery and became the biggest exporter of Mahi-mahi with multi Tai wanese seafood one of the leading exporter in other expertise in Wahoo, Mackerel, processer helping them selling Tai wan, exporting more than Blue Shark, Sword Fish, Sail Fish, King seafood to US 10,000 tons of fishery annually Fish, Tilapia...etc. 1975 1977 1990 1991-2018 Focused on shrimp business as Tai wan held Awarded ”Taiwan Excellent Exporter” for 30 the leading position in years consecutively global shrimp market Certified by HACCP, SQF, SGS and BRC, Ocean Leader Co. Ltd., demonstrated the pursuit in products sanitation and safety 2 Global Footprint Canada Europe USA Bahama UK Jamaica Dominica Puerto Rico Trinidad Mexico Ecuador 3 Products Overview Mahi Mahi Mackerel FRESH IQF Sword Fish Black Marlin King Fish Wahoo SEAFOOD Blue Shark Sail Fish CERTIFIED Golden Yellowfin Tuna Pompano Pacific Saury Oil Fish Mackerel Pike 4 Mahi Mahi Coryphaena Hippurus Product Specification: • Whole gutted • Fillet • Loin • Portion • Steak *All pictures are for illustration purpose only 5 Blue Shark Priunace Glauca Product Specification: • H+G (Headed & Gutted) • Fillet • Portion • Steak • Cube *All pictures are for illustration purpose only 6 Mackerel Scomber Japonicus Product Specification: • Whole round • Fillet *All pictures are for illustration purpose only 7