Understanding the Cancer Stem Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intratumor Heterogeneity: Novel Approaches for Resolving Genomic Architecture and Clonal Evolution Ravi G
Published OnlineFirst June 8, 2017; DOI: 10.1158/1541-7786.MCR-17-0070 Review Molecular Cancer Research Intratumor Heterogeneity: Novel Approaches for Resolving Genomic Architecture and Clonal Evolution Ravi G. Gupta and Robert A. Somer Abstract High-throughput genomic technologies have revealed a full mutational landscape of tumors could help reconstruct remarkably complex portrait of intratumor heterogeneity in their phylogenetic trees and trace the subclonal origins of cancer and have shown that tumors evolve through a reiterative therapeutic resistance, relapsed disease, and distant metastases, process of genetic diversification and clonal selection. This the major causes of cancer-related mortality. Real-time assess- discovery has challenged the classical paradigm of clonal ment of the tumor subclonal architecture, however, remains dominance and brought attention to subclonal tumor cell limited by the high rate of errors produced by most genome- populations that contribute to the cancer phenotype. Dynamic wide sequencing methods as well as the practical difficulties evolutionary models may explain how these populations grow associated with serial tumor genotyping in patients. This review within the ecosystem of tissues, including linear, branching, focuses on novel approaches to mitigate these challenges using neutral, and punctuated patterns. Recent evidence in breast bulk tumor, liquid biopsies, single-cell analysis, and deep cancer favors branching and punctuated evolution driven by sequencing techniques. The origins of intratumor heterogene- genome instability as well as nongenetic sources of heteroge- ity and the clinical, diagnostic, and therapeutic consequences neity, such as epigenetic variation, hierarchal tumor cell orga- in breast cancer are also explored. Mol Cancer Res; 15(9); 1127–37. nization, and subclonal cell–cell interactions. -

Stem Cells and Cancer
Stem Cells and Cancer Cancer Education Project Stem Cells and Cancer Overview: This series of activities is designed to introduce students to the theory that some cancers arise from cancer stem cells. This theory provides a possible explanation for why cancers reoccur after cancer treatment. It also provides insights that may lead to new types of chemotherapy drugs. • Part 1: Stem Cells and Cancer PowerPoint (40 minutes) Students view a PowerPoint presentation that introduces stem cell biology and shows ways that cancer stem cell research might lead to more effective cancer therapy treatments. Students answer questions as they view the PowerPoint. Then they create a cartoon strip to illustrate their understanding of cancer stem cells. • Part 2: The Bad Seed: Rare stem cells appear to drive cancers (20 minutes) Students read a brief article that introduces stem cell biology and explains how cancer stem cell research might lead to more effective cancer therapy treatments. Students answer questions based on this article. This activity may be done in class or for homework. • Part 3: Plant Derivative Attacks the Roots of Leukemia (20 minutes) Students read a brief article on the development of a potential chemotherapy agent that specifically targets cancer stem cells. Students answer questions based on this article. This activity may be done in class or for homework. • Part 4: Clinical Trials: Parthocet (40 minutes) Students answer questions about the design of a large-scale, randomized, double-blind clinical trial to determine if Parthocet (a fictitious chemotherapy drug) is safe and effective. Life Sciences Learning Center – Cancer Education Project 1 Copyright © 2007, University of Rochester May be copied for classroom use Stem Cells and Cancer Teacher Instructions - Part 1 Stem Cells and Cancer PowerPoint Presentation Students view a PowerPoint presentation that introduces stem cell biology and shows ways that cancer stem cell research might lead to more effective cancer therapy treatments. -

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -
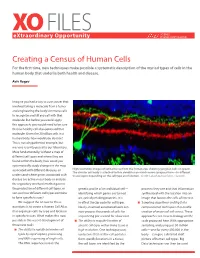
Creating a Census of Human Cells
XO FILES SCIENCE eXtraordinary Opportunity PHILANTHROPY ALLIANCE Creating a Census of Human Cells For the first time, new techniques make possible a systematic description of the myriad types of cells in the human body that underlie both health and disease. Aviv Regev Imagine you had a way to cure cancer that involved taking a molecule from a tumor and engineering the body’s immune cells to recognize and kill any cell with that molecule. But before you could apply this approach, you would need to be sure that no healthy cell also expressed that molecule. Given the 20 trillion cells in a human body, how would you do that? This is not a hypothetical example, but was one recently posed to our laboratory. More fundamentally, without a map of different cell types and where they are found within the body, how could you systematically study changes in the map associated with different diseases, or High resolution images of retinal tissue from the human eye, showing ganglion cells (in green). The circular cell body is attached to thin dendrites on which nerve synapses form—in different understand where genes associated with tissue layers depending on the cell type and function. Credit: Lab of Joshua Sanes, Harvard. disease are active in our body or analyze the regulatory mechanisms that govern the production of different cell types, or genetic profile of an individual cell— proteins they use and that information sort out how different cell types combine identifying which genes are turned synthesized with the location into an to form specific tissues? on, actively making proteins. -

Cancer Stem Cells and Nucleolin As Drivers of Carcinogenesis
pharmaceuticals Review Cancer Stem Cells and Nucleolin as Drivers of Carcinogenesis Laura Sofia Carvalho 1,Nélio Gonçalves 1 , Nuno André Fonseca 1,2 and João Nuno Moreira 1,3,* 1 CNC—Center for Neurosciences and Cell Biology, Center for Innovative Biomedicine and Biotechnology (CIBB), Faculty of Medicine (Polo 1), University of Coimbra, Rua Larga, 3004-504 Coimbra, Portugal; laurasofi[email protected] (L.S.C.); [email protected] (N.G.); [email protected] (N.A.F.) 2 TREAT U, SA—Parque Industrial de Taveiro, Lote 44, 3045-508 Coimbra, Portugal 3 UC—University of Coimbra, CIBB, Faculty of Pharmacy (FFUC), Pólo das Ciências da Saúde, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal * Correspondence: [email protected]; Tel.: +351-239-820-190 Abstract: Cancer, one of the most mortal diseases worldwide, is characterized by the gain of specific features and cellular heterogeneity. Clonal evolution is an established theory to explain heterogeneity, but the discovery of cancer stem cells expanded the concept to include the hierarchical growth and plasticity of cancer cells. The activation of epithelial-to-mesenchymal transition and its molecular players are widely correlated with the presence of cancer stem cells in tumors. Moreover, the acquisition of certain oncological features may be partially attributed to alterations in the levels, location or function of nucleolin, a multifunctional protein involved in several cellular processes. This review aims at integrating the established hallmarks of cancer with the plasticity of cancer cells as an emerging hallmark; responsible for tumor heterogeneity; therapy resistance and relapse. The discussion will contextualize the involvement of nucleolin in the establishment of cancer hallmarks and its application as a marker protein for targeted anticancer therapies Keywords: tumor heterogeneity; drug resistance; cancer stem cells; nucleolin; targeted therapies; epithelial-to-mesenchymal transition Citation: Carvalho, L.S.; Gonçalves, N.; Fonseca, N.A.; Moreira, J.N. -

Robustness and Timing of Cellular Differentiation Through Population-Based Symmetry Breaking
AR TI CLE Robustness AND TIMING OF CELLULAR DIFFERENTIATION THROUGH population-based SYMMETRY BREAKING Angel StanoeV1 | Christian Schröter1 | Aneta KOSESKA1∗ 1Department OF Systemic Cell Biology, Max Planck Institute OF Molecular PhYSIOLOGY, Although COORDINATED BEHAVIOR OF MULTICELLULAR ORGANISMS Dortmund, GermanY RELIES ON INTERCELLULAR communication, THE DYNAMICAL mech- Correspondence ANISM THAT UNDERLIES SYMMETRY BREAKING IN HOMOGENEOUS Email: populations, THEREBY GIVING RISE TO HETEROGENEOUS CELL TYPES ∗[email protected] REMAINS UNCLEAR. In CONTRAST TO THE PREVALENT cell-intrinsic VIEW OF DIFFERENTIATION WHERE MULTISTABILITY ON THE SINGLE CELL LEVEL IS A NECESSARY pre-requisite, WE PROPOSE THAT het- EROGENEOUS CELLULAR IDENTITIES EMERGE AND ARE MAINTAINED COOPERATIVELY ON A POPULATION LEVel. Identifying A GENERIC SYMMETRY BREAKING mechanism, WE DEMONSTRATE THAT ROBUST PROPORTIONS BETWEEN DIFFERENTIATED CELL TYPES ARE INTRINSIC FEATURES OF THIS INHOMOGENEOUS STEADY STATE solution. Crit- ICAL ORGANIZATION IN THE VICINITY OF THE SYMMETRY BREAKING BIFURCATION ON THE OTHER hand, SUGGESTS THAT TIMING OF cel- LULAR DIFFERENTIATION EMERGES FOR GROWING POPULATIONS IN A self-organized MANNER. Setting A CLEAR DISTINCTION BETWEEN pre-eXISTING ASYMMETRIES AND SYMMETRY BREAKING EVents, WE THUS DEMONSTRATE THAT EMERGENT SYMMETRY breaking, IN CONJUNCTION WITH ORGANIZATION NEAR CRITICALITY NECESSARILY LEADS TO ROBUSTNESS AND ACCURACY DURING DEVelopment. KEYWORDS SYMMETRY breaking; differentiation; INHOMOGENEOUS STEADY STATE Abbreviations: -

Cancer from the Perspective of Stem Cells and Misappropriated Tissue Regeneration Mechanisms
Leukemia (2018) 32:2519–2526 https://doi.org/10.1038/s41375-018-0294-7 REVIEW ARTICLE Corrected: Correction Stem cell biology Cancer from the perspective of stem cells and misappropriated tissue regeneration mechanisms 1,2 1 1 1,2 1 Mariusz Z. Ratajczak ● Kamila Bujko ● Aaron Mack ● Magda Kucia ● Janina Ratajczak Received: 8 September 2018 / Accepted: 17 September 2018 / Published online: 30 October 2018 © The Author(s) 2018. This article is published with open access Abstract Tumorigenesis can be considered as pathologically misappropriated tissue regeneration. In this review we will address some unresolved issues that support this concept. First, we will address the issue of the identity of cancer-initiating cells and the presence of cancer stem cells in growing tumors. We will also ask are there rare and distinct populations of cancer stem cells in established tumor cell lines, or are all of the cells cancer stem cells? Second, the most important clinical problem with cancer is its metastasis, and here a challenging question arises: by employing radio-chemotherapy for tumor treatment, do we unintentionally create a prometastatic microenvironment in collateral organs? Specifically, many factors upregulated in response to radio-chemotherapy-induced injury may attract highly migratory cancer cells that survived initial treatment. 1234567890();,: 1234567890();,: Third, what is the contribution of normal circulating stem cells to the growing malignancy? Do circulating normal stem cells recognize a tumor as a hypoxia-damaged tissue that needs -

A Concise Review on the Classification and Nomenclature of Stem Cells Kök Hücrelerinin S›N›Fland›R›Lmas› Ve Isimlendirilmesine Iliflkin K›Sa Bir Derleme
Review 57 A concise review on the classification and nomenclature of stem cells Kök hücrelerinin s›n›fland›r›lmas› ve isimlendirilmesine iliflkin k›sa bir derleme Alp Can Ankara University Medical School, Department of Histology and Embryology, Ankara, Turkey Abstract Stem cell biology and regenerative medicine is a relatively young field. However, in recent years there has been a tremen- dous interest in stem cells possibly due to their therapeutic potential in disease states. As a classical definition, a stem cell is an undifferentiated cell that can produce daughter cells that can either remain a stem cell in a process called self-renew- al, or commit to a specific cell type via the initiation of a differentiation pathway leading to the production of mature progeny cells. Despite this acknowledged definition, the classification of stem cells has been a perplexing notion that may often raise misconception even among stem cell biologists. Therefore, the aim of this brief review is to give a conceptual approach to classifying the stem cells beginning from the early morula stage totipotent embryonic stem cells to the unipotent tissue-resident adult stem cells, also called tissue-specific stem cells. (Turk J Hematol 2008; 25: 57-9) Key words: Stem cells, embryonic stem cells, tissue-specific stem cells, classification, progeny. Özet Kök hücresi biyolojisi ve onar›msal t›p görece yeni alanlard›r. Buna karfl›n, son y›llarda çeflitli hastal›klarda tedavi amac›yla kullan›labilme potansiyelleri nedeniyle kök hücrelerine ola¤anüstü bir ilgi art›fl› vard›r. Klasik tan›m›yla kök hücresi, kendini yenileme ad› verilen mekanizmayla farkl›laflmadan kendini ço¤altan veya bir dizi farkl›laflma aflamas›ndan geçerek olgun hücrelere dönüflebilen hücrelerdir. -

Intra-Tumor Heterogeneity from a Cancer Stem Cell Perspective Pramudita R
Prasetyanti and Medema Molecular Cancer (2017) 16:41 DOI 10.1186/s12943-017-0600-4 REVIEW Open Access Intra-tumor heterogeneity from a cancer stem cell perspective Pramudita R. Prasetyanti1,2 and Jan Paul Medema1,2,3* Abstract Tumor heterogeneity represents an ongoing challenge in the field of cancer therapy. Heterogeneity is evident between cancers from different patients (inter-tumor heterogeneity) and within a single tumor (intra-tumor heterogeneity). The latter includes phenotypic diversity such as cell surface markers, (epi)genetic abnormality, growth rate, apoptosis and other hallmarks of cancer that eventually drive disease progression and treatment failure. Cancer stem cells (CSCs) have been put forward to be one of the determining factors that contribute to intra-tumor heterogeneity. However, recent findings have shown that the stem-like state in a given tumor cell is a plastic quality. A corollary to this view is that stemness traits can be acquired via (epi)genetic modification and/or interaction with the tumor microenvironment (TME). Here we discuss factors contributing to this CSC heterogeneity and the potential implications for cancer therapy. Keywords: Tumor heterogeneity, Cancer stem cell, Stemness, Tumor microenvironment Background tumor and microenvironment heterogeneity determine A tumor is a heterogeneous population of cells, containing the fitness of the tumor and as such, are likely to be transformed cancer cells, supportive cells and tumor- crucial factors in treatment success. infiltrating cells. This intra-tumor heterogeneity is further Two models have been proposed to account for hetero- enhanced by clonal variation and microenvironmental geneity within a tumor. In the clonal evolution model, influences on cancer cells, which also do not represent a stochastic mutations in individual tumor cells serve as homogeneous set of cells. -

Cancer Stem Cells: a New Approach to Tumor Development
REVIEW ARTICLE KOBAYASHI NCC ET AL. Cancer stem cells: a new approach to tumor development NATÁLIA CRISTINA CIUFA KOBAYASHI1*, SAMUEL MARCOS RIBEIRO DE NORONHA2 1Full Teaching Degree in Biological Sciences – Graduate degree in Molecular Biology, United Metropolitan Colleges (FMU), São Paulo, SP, Brazil 2PhD – Department of Allergy and Immunology, University of São Paulo (USP), São Paulo, SP, Brazil SUMMARY Many theories have been proposed to explain the origins of cancer. Currently, evidences show that not every tumor cell is capable of initiating a tumor. Only a small part of the cancer cells, called cancer stem cells (CSCs), can generate a tumor identical to the original one, when removed from human tumors and transplanted into immunosuppressed mice. The name given to these cells co- mes from the resemblance to normal stem cells, except for the fact that their abi- lity to divide is infinite. These cells are also affected by their microenvironment. Many of the signaling pathways, such as Wnt, Notch and Hedgehog, are altered in this tumoral subpopulation, which also contributes to abnormal prolifera- tion. Researchers have found several markers for CSCs; however, much remains to be studied, or perhaps a universal marker does not even exist, since they vary Study conducted at United among tumor types and even from patient to patient. It was also found that Metropolitan Colleges (FMU) cancer stem cells are resistant to radiotherapy and chemotherapy. This may ex- Article received: 4/24/2014 plain the re-emergence of the disease, since they are not completely eliminated Accepted for publication: 4/24/2014 and minimal amounts of CSCs can repopulate a tumor. -

Advances in Therapeutic Targeting of Cancer Stem Cells Within the Tumor Microenvironment: an Updated Review
cells Review Advances in Therapeutic Targeting of Cancer Stem Cells within the Tumor Microenvironment: An Updated Review 1,2, , 1,2, 1,2, Kevin Dzobo * y , Dimakatso Alice Senthebane y, Chelene Ganz y, Nicholas Ekow Thomford 3,4 , Ambroise Wonkam 3 and Collet Dandara 3 1 International Centre for Genetic Engineering and Biotechnology (ICGEB), Cape Town Component, Wernher and Beit Building (South), UCT Medical Campus, Anzio Road, Observatory, Cape Town 7925, South Africa; [email protected] (D.A.S.); [email protected] (C.G.) 2 Division of Medical Biochemistry and Institute of Infectious Disease and Molecular Medicine, Department of Integrative Biomedical Sciences, Faculty of Health Sciences, University of Cape Town, Cape Town 7925, South Africa 3 Division of Human Genetics, Department of Pathology and Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Cape Town 7925, South Africa; [email protected] (N.E.T.); [email protected] (A.W.); [email protected] (C.D.) 4 Department of Medical Biochemistry, School of Medical Sciences, College of Health Sciences, University of Cape Coast, PMB, Cape Coast, Ghana * Correspondence: [email protected]; Tel.: +27-842953708 These authors contributed equally to this work. y Received: 7 July 2020; Accepted: 11 August 2020; Published: 13 August 2020 Abstract: Despite great strides being achieved in improving cancer patients’ outcomes through better therapies and combinatorial treatment, several hurdles still remain due to therapy resistance, cancer recurrence and metastasis. Drug resistance culminating in relapse continues to be associated with fatal disease. The cancer stem cell theory posits that tumors are driven by specialized cancer cells called cancer stem cells (CSCs). -

Cancer Cell CD44 Mediates Macrophage/Monocyte-Driven Regulation of Head and Neck Cancer Stem Cells
Author Manuscript Published OnlineFirst on August 14, 2020; DOI: 10.1158/0008-5472.CAN-20-1079 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Article Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Authors Karina E. Gomez1, FangLong Wu2,3, Stephen B. Keysar1, J. Jason Morton1, Bettina Miller1, Tugs-Saikhan Chimed1, Phuong N. Le1, Cera Nieto1, Farshad N. Chowdhury1, Anit Tyagi1, Traci R. Lyons1,4, Christian D. Young2, Hongmei Zhou3, Hilary L. Somerset2, Xiao-Jing Wang2,4,5, and Antonio Jimeno1,4. Affiliation 1 Division of Medical Oncology, Department of Medicine, University of Colorado Anschutz Medical Campus (CU AMC), CO, 80045. 2 Department of Pathology, CU AMC, CO, 80045. 3 State Key Laboratory of Oral Diseases, Department of Oral Medicine, West China Hospital of Stomatology, Sichuan University, Chengdu, China. 4 Gates Center for Regenerative Medicine, CU AMC, CO, 80045. 5 Veterans Affairs Medical Center, VA Eastern Colorado Health Care System, Aurora, CO, USA. Corresponding Author Antonio Jimeno M.D., Ph.D., Professor of Medicine/Oncology, and Otolaryngology. University of Colorado Cancer Center, and Gates Center for Regenerative Medicine. University of Colorado Anschutz Medical Campus. 12801 East 17th Avenue, Room L18-8101B, Aurora, CO 80045, USA. [email protected] 1 Downloaded from cancerres.aacrjournals.org on September 28, 2021. © 2020 American Association for Cancer Research. Author Manuscript Published OnlineFirst on August 14, 2020; DOI: 10.1158/0008-5472.CAN-20-1079 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited.