Benthic-Pelagic Coupling in Coastal Seas – Modelling Macrofaunal Biomass and Carbon Processing in Response to Organic Matter S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Lagoon cockle (Cerastoderma glaucum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2002-07-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1315]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2002. Cerastoderma glaucum Lagoon cockle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1315.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-07-15 Lagoon cockle (Cerastoderma glaucum) - Marine Life Information Network See online review for distribution map Three Cerastoderma glaucum with siphons extended. Distribution data supplied by the Ocean Photographer: Dennis R. -

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

Articles (Newman and Unger, 2003; Rainbow, 1995; Cumulation in Shells
Biogeosciences, 18, 707–728, 2021 https://doi.org/10.5194/bg-18-707-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. The patterns of elemental concentration (Ca, Na, Sr, Mg, Mn, Ba, Cu, Pb, V, Y, U and Cd) in shells of invertebrates representing different CaCO3 polymorphs: a case study from the brackish Gulf of Gdansk´ (the Baltic Sea) Anna Piwoni-Piórewicz1, Stanislav Strekopytov2,a, Emma Humphreys-Williams2, and Piotr Kuklinski´ 1,3 1Institute of Oceanology, Polish Academy of Sciences, Sopot, 81-712, Poland 2Imaging and Analysis Centre, Natural History Museum, London, SW7 5BD, United Kingdom 3Department of Life Sciences, Natural History Museum, London, SW7 5BD, United Kingdom acurrent address: National Measurement Laboratory, LGC Limited, Teddington, TW11 0LY, United Kingdom Correspondence: Anna Piwoni-Piórewicz ([email protected]) Received: 10 September 2019 – Discussion started: 20 September 2019 Revised: 6 November 2020 – Accepted: 9 December 2020 – Published: 29 January 2021 Abstract. The shells of calcitic arthropod Amphibalanus 1 Introduction improvisus; aragonitic bivalves Cerastoderma glaucum, Limecola balthica, and Mya arenaria; and bimineralic bi- Marine invertebrates such as molluscs, brachiopods, corals, valve Mytilus trossulus were collected in the brackish waters echinoderms, bryozoans and some groups of protozoa of the southern Baltic Sea in order to study patterns of bulk (foraminifera) are able to form skeletons built of calcium car- elemental concentration (Ca, Na, Sr, Mg, Ba, Mn, Cu, Pb, V, bonate. The combination of inorganic CaCO3 crystals and or- Y, U and Cd) in shells composed of different crystal lattices ganic compounds with a characteristic and ordered structure, (calcite and aragonite). -

Outer Ards Modiolus Modiolus Report
R Assessment of Outer Ards Modiolus modiolus biogenic reefs against Special Area of Conservation (SAC) criteria JULY 2016 A report from the Fisheries and Aquatic Ecosystems Branch, Agri-food and Biosciences Institute to The Department of Agriculture, Environment and Rural Affairs (Northern Ireland) Document version control: Version Issue date Modifier Note Issued to and date 1.0 31/03/2016 AFBI-AC First draft for review DAERA & MS: 31/03/2016 1.1 19/05/2016 AFBI-AC Second draft for review DAERA & MS: 19/05/2016 1.2 19/07/2016 AFBI-AC Final draft for sign off following MS: 19/07/2016 receipt of comments 22/06/2016 1.3 19/07/2016 AFBI-AC Final version DAERA: 20/07/2016 Further information Dr. Annika Clements Seabed Habitat Mapping Project Leader Fisheries & Aquatic Ecosystems Branch Newforge Lane Belfast BT9 5PX Tel: +44(0)2890255153 Email: [email protected] DAERA Client Officer Joe Breen DAERA, Marine Conservation and Reporting Team Marine & Fisheries Division Portrush Coastal Zone 8 Bath Road PORTRUSH BT56 8AP Tel: +44(0)2870823600 (ext31) Email: [email protected] The GIS project “Outer_Ards_Modiolus_2016.mxd” should be available for use in conjunction with this report. Recommended citation: AFBI, 2016. Special Area of Conservation Designation Assessment of Outer Ards Modiolus modiolus Biogenic Reef. Report to the Department of Agriculture, Environment and Rural Affairs, Northern Ireland. Acknowledgements The author wishes to thank Adele Boyd and Matthew Service (AFBI) for data provision, James McArdle (AFBI), Katie Lilley (Ulster University placement student with AFBI) and Clara Alvarez Alonso (DAERA) and the master and crew of the R.V. -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

Cellular Level Response of the Bivalve Limecola Balthica To
Science of the Total Environment 794 (2021) 148593 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Cellular level response of the bivalve Limecola balthica to seawater acidification due to potential CO2 leakage from a sub-seabed storage site in the southern Baltic Sea: TiTank experiment at representative hydrostatic pressure Adam Sokołowski a,1, Justyna Świeżak a,⁎,1, Anna Hallmann b, Anders J. Olsen c, Marcelina Ziółkowska a, Ida Beathe Øverjordet d, Trond Nordtug d, Dag Altin e, Daniel Franklin Krause d, Iurgi Salaberria c, Katarzyna Smolarz a a University of Gdańsk, Faculty of Oceanography and Geography, Institute of Oceanography, Al. Piłsudskiego 46, 81-378 Gdynia, Poland b Medical University of Gdańsk, Department of Pharmaceutical Biochemistry, Dębinki 1, 80-211 Gdańsk, Poland c Norwegian University of Science and Technology, NO-7491 Trondheim, Norway d SINTEF Ocean AS, Brattorkaia 17C, NO-7465 Trondheim, Norway e Altins Biotrix, Finn Bergs veg 3, 7022 Trondheim, Norway HIGHLIGHTS GRAPHICAL ABSTRACT • Cellular level responses of L. balthica to acidification caused by CO2 was tested at 9 ATM pressure. • The bivalve is tolerant to medium-term severe environmental hypercapnia. • Seawater pH 7.0 induced effects on rad- ical defence mechanisms (GPx, GST, CAT). • pH 6.3 caused increased cellular oxida- tive stress (MDA) and detoxification (tGSH). article info abstract Article history: Understanding of biological responses of marine fauna to seawater acidification due to potential CO2 leakage Received 7 April 2021 from sub-seabed storage sites has improved recently, providing support to CCS environmental risk assessment. Received in revised form 15 June 2021 Physiological responses of benthic organisms to ambient hypercapnia have been previously investigated but Accepted 17 June 2021 rarely at the cellular level, particularly in areas of less common geochemical and ecological conditions such as Available online 24 June 2021 brackish water and/or reduced oxygen levels. -

GENETIC STRUCTURE of the LIMECOLA BALTHICA POPULATION in the GULF of RIGA, BALTIC SEA Oksana Fokina1, Dace Grauda1, Ingrîda Puriòa2, Ieva Bârda2, and Isaak Rashal1
PROCEEDINGS OF THE LATVIAN ACADEMY OF SCIENCES. Section B, Vol. 74 (2020), No. 6 (729), pp. 381–384. DOI: 10.2478/prolas-2020-0057 GENETIC STRUCTURE OF THE LIMECOLA BALTHICA POPULATION IN THE GULF OF RIGA, BALTIC SEA Oksana Fokina1, Dace Grauda1, Ingrîda Puriòa2, Ieva Bârda2, and Isaak Rashal1 1 Institute of Biology, University of Latvia, 1 Jelgavas Str., Rîga, LV-1004, LATVIA 2 Latvian Institute of Aquatic Ecology, 4 Voleru Str., Rîga, LV-1007, LATVIA # Corresponding author, [email protected] Contributed by Isaak Rashal Samples of Limecola balthica with normal and deformed shells were collected from ten sites throughout the Gulf of Riga. Genetic diversity was evaluated by the retrotransposon-based iPBS method. Samples had close mutual genetic distances, which showed that all of them belong to one wider population of the Gulf of Rîga. No direct relationship between the activity of retro- transposons and deformation of shells was found. Key words: retrotransposon-based markers, iPBS, Baltic macoma, genetic distances. INTRODUCTION nation of several factors. According to Sokolowski et al. (2004), the number of deformed shells of L. balthica from Baltic Sea is the world’s largest brackish sea and has re- the Gulf of Gdansk increases with the depth. The concentra- stricted water exchange with the North Sea. An increased tion of accumulated trace metals (e.g., As, Ag, Cu, and Zn) level of eutrophication and pollution from hazardous sub- in the tissues might also cause deformations. The proportion stances has caused the Baltic Sea to be classified as one of of modified shells can reach up to 65% of the total popula- the most polluted areas of the world (Smolarz and Bradtke, tion size. -

HETA ROUSI: Zoobenthos As Indicators of Marine Habitats in the Northern Baltic
Heta Rousi Zoobenthos as indicators of marine Heta Rousi | habitats in the northern Baltic Sea of marine as indicators habitats in the northernZoobenthos Baltic Sea Heta Rousi This thesis describes how physical and chemical environmental variables impact zoobenthic species distribution in the northern Baltic Sea and how dis- Zoobenthos as indicators of marine tinct zoobenthic species indicate different marine benthic habitats. The thesis inspects the effects of habitats in the northern Baltic Sea depth, sediment type, temperature, salinity, oxy- gen, nutrients as well as topographical and geo- logical factors on zoobenthos on small and large temporal and spatial scales. | 2020 ISBN 978-952-12-3944-1 Heta Rousi Född 1979 Studier och examina Magister vid Helsingfors Universitet 2006 Licentiat vid Åbo Akademi 2013 Doktorsexamen vid Åbo Akademi 2020 Institutionen för miljö- och marinbiologi, Åbo Akademi ZOOBENTHOS AS INDICATORS OF MARINE HABITATS IN THE NORTHERN BALTIC SEA HETA ROUSI Environmental and Marine Biology Faculty of Science and Engineering Åbo Akademi University Finland, 2020 SUPERVISED BY PRE-EXAMINED BY Professor Erik Bonsdorff Research Professor (Supervisor & Examiner) Markku Viitasalo Åbo Akademi University Finnish Environment Institute Faculty of Science and Engineering Sustainable Use of the Marine Areas Environmental and Marine Biology Latokartanonkaari 11 Artillerigatan 6 00790 Helsinki 20520 Åbo Finland Finland Professor Emeritus Ilppo Vuorinen CO-SUPERVISOR University of Turku Adjunct Professor Faculty of Science and Engineering Samuli Korpinen Itäinen Pitkäkatu 4 Finnish Environment Institute 20520 Turku Marine Management Finland Latokartanonkaari 11 00790 Helsinki FACULTY OPPONENT Finland Associate Professor Urszula Janas SUPERVISING AT THE University of Gdansk LICENCIATE PHASE Institute of Oceanography Assistant Professor Al. -

Download and Streaming), and Products (Analytics and Indexes)
BOOK OF ABSTRACTS 53RD EUROPEAN MARINE BIOLOGY SYMPOSIUM OOSTENDE, BELGIUM 17-21 SEPTEMBER 2018 This publication should be quoted as follows: Mees, J.; Seys, J. (Eds.) (2018). Book of abstracts – 53rd European Marine Biology Symposium. Oostende, Belgium, 17-21 September 2018. VLIZ Special Publication, 82. Vlaams Instituut voor de Zee - Flanders Marine Institute (VLIZ): Oostende. 199 pp. Vlaams Instituut voor de Zee (VLIZ) – Flanders Marine Institute InnovOcean site, Wandelaarkaai 7, 8400 Oostende, Belgium Tel. +32-(0)59-34 21 30 – Fax +32-(0)59-34 21 31 E-mail: [email protected] – Website: http://www.vliz.be The abstracts in this book are published on the basis of the information submitted by the respective authors. The publisher and editors cannot be held responsible for errors or any consequences arising from the use of information contained in this book of abstracts. Reproduction is authorized, provided that appropriate mention is made of the source. ISSN 1377-0950 Table of Contents Keynote presentations Engelhard Georg - Science from a historical perspective: 175 years of change in the North Sea ............ 11 Pirlet Ruth - The history of marine science in Belgium ............................................................................... 12 Lindeboom Han - Title of the keynote presentation ................................................................................... 13 Obst Matthias - Title of the keynote presentation ...................................................................................... 14 Delaney Jane - Title -
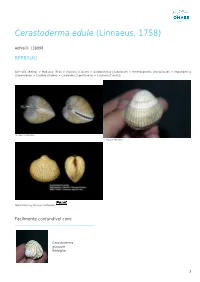
Cerastoderma Edule (Linnaeus, 1758)
Cerastoderma edule (Linnaeus, 1758) AphiaID: 138998 BERBIGÃO Animalia (Reino) > Mollusca (Filo) > Bivalvia (Classe) > Autobranchia (Subclasse) > Heteroconchia (Infraclasse) > Imparidentia (Superordem) > Cardiida (Ordem) > Cardioidea (Superfamilia) > Cardiidae (Familia) © Vasco Ferreira © Vasco Ferreira Natural History Museum Rotterdam Facilmente confundível com: Cerastoderma glaucum Berbigão 1 Estatuto de Conservação Sinónimos Cardium belgicum De Malzine, 1867 Cardium crenulatum Lamarck, 1819 Cardium edule Linnaeus, 1758 Cardium edule burchanae Girscher, 1938 Cardium edule var. batesoni Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. loppensi Mars, 1951 Cardium edule var. maculata Dautzenberg, 1890 Cardium edule var. major Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. mareotica Pallary, 1912 Cardium edule var. regularis Pallary, 1900 Cardium edule var. sibenicensis Brusina, 1870 Cardium mercatorium Coen, 1915 Cardium nunninkae Lucas, 1984 Cardium obtritum Locard, 1886 Cardium quadrarium Reeve, 1845 Cardium vulgare da Costa, 1778 Cardium vulgatum Tryon, 1872 Cerastoderma edule var. sinicola Lacourt, 1974 Cerastoderma nunninkae Lucas, 1984 Referências basis of record Gofas, S.; Le Renard, J.; Bouchet, P. (2001). Mollusca. in: Costello, M.J. et al. (eds), European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Patrimoines Naturels. 50: 180-213. [details] additional source Poorten, J.J. ter, 2005. Outline of a systematic index – Recent Cardiidae (Lamarck, 1809). VISAYA net. (Updated 2009 for WoRMS), available online at http://www.conchology.be/en/shelltopics/visaya-net/date.php?year=2005 [details] 2 ecology source Coscia, I., P.E. Robins, J.S. Porter, S.K. Malham & J.E. Ironside. (2013). Modelled larval dispersal and measured gene flow: seascape genetics of the common cockle Cerastoderma edule in the southern Irish Sea. -

Sample Size Effects on the Assessment of Eukaryotic Diversity
www.nature.com/scientificreports OPEN Sample size efects on the assessment of eukaryotic diversity and community structure in aquatic Received: 26 January 2018 Accepted: 23 July 2018 sediments using high-throughput Published: xx xx xxxx sequencing Francisco J. A. Nascimento 1,4, Delphine Lallias2, Holly M. Bik 3 & Simon Creer 4 Understanding how biodiversity changes in time and space is vital to assess the efects of environmental change on benthic ecosystems. Due to the limitations of morphological methods, there has been a rapid expansion in the application of high-throughput sequencing methods to study benthic eukaryotic communities. However, the efect of sample size and small-scale spatial variation on the assessment of benthic eukaryotic diversity is still not well understood. Here, we investigate the efect of diferent sample volumes in the genetic assessment of benthic metazoan and non-metazoan eukaryotic community composition. Accordingly, DNA was extracted from fve diferent cumulative sediment volumes comprising 100% of the top 2 cm of fve benthic sampling cores, and used as template for Ilumina MiSeq sequencing of 18 S rRNA amplicons. Sample volumes strongly impacted diversity metrics for both metazoans and non-metazoan eukaryotes. Beta-diversity of treatments using smaller sample volumes was signifcantly diferent from the beta-diversity of the 100% sampled area. Overall our fndings indicate that sample volumes of 0.2 g (1% of the sampled area) are insufcient to account for spatial heterogeneity at small spatial scales, and that relatively large percentages of sediment core samples are needed for obtaining robust diversity measurement of both metazoan and non-metazoan eukaryotes. -

Elemental Composition of Invertebrates Shells Composed Of
https://doi.org/10.5194/bg-2019-367 Preprint. Discussion started: 20 September 2019 c Author(s) 2019. CC BY 4.0 License. 1 Elemental composition of invertebrates shells composed of different CaCO3 polymorphs at 2 different ontogenetic stages: a case study from the brackish Gulf of Gdansk (the Baltic Sea) 3 4 Anna Piwoni-Piórewicz1*, Stanislav Strekopytov2†, Emma Humphreys-Williams2, Piotr 5 Kukliński1, 3 6 7 1Institute of Oceanology, Polish Academy of Sciences, Powstańców Warszawy 55, 81-712 8 Sopot, Poland 9 2Imaging and Analysis Centre, Natural History Museum, Cromwell Road, London SW7 5BD, 10 United Kingdom 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, 12 United Kingdom 13 14 *Corresponding author 15 E-mail: [email protected] 16 Tel.: (+ 48 58) 731 16 96 17 † Present address: Inorganic Analysis, LGC Ltd, Queens Road, Teddington, United Kingdom 18 19 Abstract 20 In this study, the concentrations of 12 metals: Ca, Na, Sr, Mg, Ba, Mn, Cu, Pb, V, Y, U and 21 Cd in shells of bivalve molluscs (aragonitic: Cerastoderma glaucum, Mya arenaria and 22 Limecola balthica and bimineralic: Mytilus trossulus) and arthropods (calcitic: Amphibalanus 23 improvisus) were obtained. The main goal was to determine the incorporation patterns of 24 shells built with different calcium carbonate polymorphs. The role of potential biological 25 control on the shell chemistry was assessed by comparing the concentrations of trace elements 26 between younger and older individuals (different size classes). The potential impact of 27 environmental factors on the observed elemental concentrations in the studied shells is 28 discussed.