Clinical Policy: Infertility and Fertility Preservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Chorionic Gonadotropin (HCG), a Polypeptide Hormone Produced by the Human
45792G/Revised: April 2011 CHORIONIC GONADOTROPIN FOR INJECTION, USP DESCRIPTION: Human chorionic gonadotropin (HCG), a polypeptide hormone produced by the human placenta, is composed of an alpha and a beta sub-unit. The alpha sub-unit is essentially identical to the alpha sub-units of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha sub-unit of human thyroid-stimulating hormone (TSH). The beta sub-units of these hormones differ in amino acid sequence. Chorionic gonadotropin is obtained from the human pregnancy urine. It is standardized by a biological assay procedure. Chorionic Gonadotropin for Injection, USP is available in multiple dose vials containing 10,000 USP Units with accompanying Bacteriostatic Water for Injection for reconstitution. When reconstituted with 10 mL of the accompanying diluent each vial contains: Chorionic gonadotropin 10,000 Units Mannitol 100 mg Benzyl alcohol 0.9% Water for Injection q.s. Buffered with dibasic sodium phosphate and monobasic sodium phosphate. Hydrochloric acid and/or sodium hydroxide may have been used for pH adjustment (6.0 Reference ID: 2933198 8.0). Nitrogen gas is used in the freeze drying process. CLINICAL PHARMACOLOGY: The action of HCG is virtually identical to that of pituitary LH, although HCG appears to have a small degree of FSH activity as well. It stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce androgens and the corpus luteum of the ovary to produce progesterone. Androgen stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. -

Gonadotropin and Testosterone Measurements After
Pediat. Res. 10: 46-51 (1976) Estradiol puberty estrogen sexual differentiation gonadotropins testosterone maturation Gonadotropin and Testosterone Measurements after Estrogen Administration to Adult Men, Prepubertal and Pubertal Boys, and Men with Hypogonadotropism: Evidence for Maturation of Positive Feedback in the Male HOWARD E. KULIN"" AND EDWARD 0. REITER Reproduction Research Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA Extract MATERIALS AND METHODS Nineteen male subjects were given five daily injections of SUBJECTS 17~-estradiol and circulating levels of estradiol (E 2 ), testosterone (T), and gonadotropins were determined by radioimmunoassay Seven normal, adult men (ages 20-22) and one male (age 21) before, during, and after the steroid course. Peak levels of E 2 with the syndrome of vanishing testes (I) were hospitalized for attained during the 5 days of treatment ranged from 173-577 pg/ml. study at the Clinical Center of the National Institutes of Health. Four of seven normal adult men and one castrate man demonstrated Each patient was given five daily intramuscular injections of IO or suppression of follicle-stimulating hormone ( FSH) and luteinizing 15 ,ug/kg body weight of 17~-estradiol (E2 ) (20) and blood samples hormone ( LH) with a subsequent rise in LH ( positive feedback) were obtained every 12-24 hr before, during, and after the estrogen while E 2 levels remained elevated. A rise in T was associated with course (see Table I). the LH increment in the four normal men. Nine pre-, early, or Nine endocrinologically normal boys between the ages of 7 and midpubertal boys and two men with hypogonadotropic hypogo 18 were studied in the course of evaluation for short stature, nadism displayed only gonadotropin suppression after E 2 adminis precocious puberty, or delayed adolescence. -

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -

Chorionic Gonadotropin Human (C0684)
Chorionic gonadotropin human Product Number C 0684 Storage Temperature -0 °C Product Description When hCG was used in combination with recombinant CAS Number: 9002-61-3 interferon-γ, there was a significant cooperative pI = 2.951 induction of nitric oxide synthesis (iNOS) in a dose- Extinction Coefficient: E1% = 3.88 (278nm)2 dependent manner in mouse peritoneal macrophages Synonym: Choriogonin, hCG suggesting that hCG may provide a second signal for synergistic induction of NO synthesis.9 The molecular weight is approximately 37.9 kDa (with approximately 31% carbohydrate by weight). The Precautions and Disclaimer theoretical molecular weight is 37.9 kDa based on the For Laboratory Use Only. Not for drug, household or native form, which contains 2 subunits. The α subunit other uses. has a molecular weight of 14.9 kDa of which approximately 10.2 kDa is for the polypeptide and Preparation Instructions approximately 4.7 kDa for the carbohydrate. The hCG is soluble in water and aqueous buffers such β subunit has a molecular weight of 23 kDa of which phosphate buffer. hCG is also soluble in aqueous approximately 16.0 kDa is for the polypeptide and glycerol and glycols and is insoluble in ethanol.1 approximately 7.0 kDa is for the carbohydrate.3,4,5 Solutions should be sterile filtered and not autoclaved. Product Number C 0684 is sterile filtered and contains Storage/Stability approximately 1,000 I.U. per vial. Dilute aqueous solutions undergo rapid loss of activity when stored frozen, or heated, or if excess acid or hCG is a glycoprotein hormone produced by the base is added. -

Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry
Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be submitted within 90 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit electronic comments to https://www.regulations.gov. Submit written comments to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. For questions regarding this draft document, contact Elaine Chang at 240-402-2628. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) July 2019 Clinical/Medical Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353; Email: [email protected] https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation -

WSAVA List of Essential Medicines for Cats and Dogs
The World Small Animal Veterinary Association (WSAVA) List of Essential Medicines for Cats and Dogs Version 1; January 20th, 2020 Members of the WSAVA Therapeutic Guidelines Group (TGG) Steagall PV, Pelligand L, Page SW, Bourgeois M, Weese S, Manigot G, Dublin D, Ferreira JP, Guardabassi L © 2020 WSAVA All Rights Reserved Contents Background ................................................................................................................................... 2 Definition ...................................................................................................................................... 2 Using the List of Essential Medicines ............................................................................................ 2 Criteria for selection of essential medicines ................................................................................. 3 Anaesthetic, analgesic, sedative and emergency drugs ............................................................... 4 Antimicrobial drugs ....................................................................................................................... 7 Antibacterial and antiprotozoal drugs ....................................................................................... 7 Systemic administration ........................................................................................................ 7 Topical administration ........................................................................................................... 9 Antifungal drugs ..................................................................................................................... -

The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men
ORIGINAL ARTICLE Endocrine Research The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men M. Y. Roth, S. T. Page, K. Lin, B. D. Anawalt, A. M. Matsumoto, B. Marck, W. J. Bremner, and J. K. Amory Downloaded from https://academic.oup.com/jcem/article/96/4/1175/2720870 by guest on 02 October 2021 Departments of Internal Medicine (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.) and Obstetrics and Gynecology (K.L.) and Center for Research in Reproduction and Contraception (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.), University of Washington, Seattle, Washington 91895; and Geriatric Research (B.M.), Education and Clinical Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington 98105 Introduction: Concentrations of intratesticular (IT) testosterone (T) are known to be 100–200 times those of serum T; however, the IT concentrations of T’s precursors, their testicular to serum gra- dients, gonadotropin dependence, and response to stimulation with human chorionic gonado- tropin (hCG) have not been studied in detail. We hypothesized that serum and IT androstenedione (ADD) and IT dehydroepiandrosterone (DHEA) would be significantly suppressed by the adminis- tration of a GnRH antagonist and increased when stimulated by hCG, without a similar suppression of serum DHEA. Methods: We suppressed gonadotropins in 23 normal men with the GnRH antagonist acyline and randomly assigned them to one of four doses of hCG, 0, 15, 60, or 125 IU sc every other day for 10 d. -

Educational Objectives Current IVF Practice
2/23/2015 Update from the IVF Lab: Preimplantation Genetic Testing Janet McLaren Bouknight, MD Reproductive Endocrinology and Infertility Department of Obstetrics & Gynecology Educational Objectives • Gain familiarity with the procedures and pregnancy outcomes of current in vitro fertilization practices. • Understand the application of Preimplantation Genetic Diagnosis (PGD) for single‐gene disorders. • Appreciate the role of Preimplantation Genetic Screening (PGS) in maximizing IVF cycle efficiency and encouraging elective single embryo transfer. Current IVF Practice 1 2/23/2015 Current IVF Practice 150,000 cycles 65,000 livebirths Current IVF Practice Current IVF Practice All SART Clinics 2012 <35 35‐37 38‐40 41‐42 >42 Pregnancy Rate 47% 38% 30% 20% 9% Live Birth Rate 41% 31% 22% 12% 4% # embryos transfer 1.9 2.0 2.4 2.9 2.9 % Twins 30% 25% 20% 13% 9% • What are the advanced that have contributed to an increase in success in the IVF Lab? • Improved lab conditions • Extended embryo culture • Improvements in embryo cryopreservation 2 2/23/2015 Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions 3 2/23/2015 Current IVF Practice: Extended Embryo Culture Day 3: Cleavage Stage Day 5: Blastocyst Stage • 8‐cells • 200‐300 cells • 30% implantation rate • 50% implantation rate Increased live birth rates seen in “good prognosis” patients who undergo a Day 5 transfer Current IVF Practice: Extended Embryo Culture Current IVF Practice: Extended Embryo Culture • Supports blastocyst transfer for “good prognosis” patients • Opportunity to consider single embryo transfer • May be associated with a small increased risk of monozygotic twinning 4 2/23/2015 Current IVF Practice: Embryo Cryopreservation Cycles • Improvements in freezing techniques (vitrification) have increased success of cryopreserved embryos. -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -
The Surrogacy Plan Plus Estimating the Costs
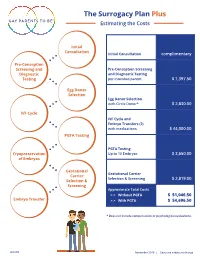
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy.