A Review of Central Retinal Artery Occlusion: Clinical Presentation And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Un-Explained Visual Loss Following Silicone Oil Removal
Roca et al. Int J Retin Vitr (2017) 3:26 DOI 10.1186/s40942-017-0079-6 International Journal of Retina and Vitreous ORIGINAL ARTICLE Open Access Un‑explained visual loss following silicone oil removal: results of the Pan American Collaborative Retina Study (PACORES) Group Jose A. Roca1, Lihteh Wu2* , Maria Berrocal3, Francisco Rodriguez4, Arturo Alezzandrini5, Gustavo Alvira6, Raul Velez‑Montoya7, Hugo Quiroz‑Mercado7, J. Fernando Arevalo8, Martín Serrano9, Luiz H. Lima10, Marta Figueroa11, Michel Farah10 and Giovanna Chico1 Abstract Purpose: To report the incidence and clinical features of patients that experienced un-explained visual loss following silicone oil (SO) removal. Methods: Multicenter retrospective study of patients that underwent SO removal during 2000–2012. Visual loss of 2 lines was considered signifcant. ≥ Results: A total of 324 eyes of 324 patients underwent SO removal during the study period. Forty two (13%) eyes sufered a signifcant visual loss following SO removal. Twenty three (7.1%) of these eyes lost vision secondary to known causes. In the remaining 19 (5.9%) eyes, the loss of vision was not explained by any other pathology. Eleven of these 19 patients (57.9%) were male. The mean age of this group was 49.2 16.4 years. Eyes that had an un-explained visual loss had a mean IOP while the eye was flled with SO of 19.6 6.9 mm± Hg. The length of time that the eye was flled with SO was 14.8 4.4 months. In comparison, eyes that± did not experience visual loss had a mean IOP of 14 7.3 mm Hg (p < 0.0002)± and a mean tamponade duration of 9.3 10.9 months (p < 0.0001). -

Fundus Signs in Temporal Arteritis
Br J Ophthalmol: first published as 10.1136/bjo.62.9.591 on 1 September 1978. Downloaded from British Journal of Ophthalmology, 1978, 62, 591-594 Fundus signs in temporal arteritis D. McLEOD, E. 0. OJI, E. M. KOHNER, AND J. MARSHALL From Moorfields Eye Hospital and Institute of Ophthalmology, London SUMMARY A patient with temporal arteritis developed a variety of ischaemic lesions in the eyes. Infarction of the inner retina and optic nerve head was delineated on presentation by white swelling in the retinal nerve fibre layer. The role of interrupted axoplasmic transport in the production of this sign is discussed. Outer retinal infarction was also noted on presentation and subsequently gave rise to striking pigmented scars. Temporal arteritis often presents with visual loss, the central venous tributaries were of normal and necropsy examination in such cases shows wide- calibre and colour. No abnormality of the inner spread disease of the ophthalmic artery and the retina was noted in the territory of supply of the extraocular course of its ciliary and retinal branches central retinal artery. (Henkind et al., 1970). The medial and lateral At first sight the left eye showed a similar ophthal- posterior ciliary arteries supply the optic nerve head, moscopic picture, with pale swelling of the nasal the outer retina, and, in 20 to 50% of individuals, part of the optic disc and a row of fluffy white by copyright. a variable area of inner retina contiguous with the cotton-wool spots crossing the papillomacular optic disc (Hayreh, 1969); the central retinal artery bundle (Fig. 2). -

Microsurgical Anatomy of the Central Retinal Artery
Original Article Microsurgical Anatomy of the Central Retinal Artery Matias Baldoncini1,2, Alvaro Campero2,3, Gabriel Moran1, Maximiliano Avendan˜ o1, Pablo Hinojosa-Martı´nez1, Marcela Cimmino1, Pablo Buosi1, Valeria Forlizzi1, Joaquı´n Chuang1, Brian Gargurevich1 - BACKGROUND: The central retinal artery (CRA) has INTRODUCTION been described as one of the first branches of the he central retinal artery (CRA) is described as one of the ophthalmic artery.It arises medial to the ciliary ganglion first branches of the ophthalmic artery. It arises medial to and after a sinuous path within the orbital cavity it pene- T the ciliary ganglion, and after a sinuous path inside the trates the lower surface of the dura mater that covers the orbital cavity, penetrates the lower surface of the dura mater that optic nerve, approximately 1 cm behind the eyeball. How- covers the optic nerve, approximately 1 cm behind the eyeball. ever, the numerous anatomic descriptions that were made After a short journey inside this meninge, it crosses the cranial of the CRA have been insufficient or unclear in relation to nerve to be located in its center and travels until it reaches the optical papilla where it divides into several branches. During this certain characteristics that are analyzed in the present entire journey, the CRA does not present any anastomoses, study. considering it as a terminal branch.1-4 However, the descriptions that many investigators make about - METHODS: An electronic literature search was made in some of thesecharacteristics of the CRA differ with the classic the PubMed database and a cadaver dissection was per- disposition] (Tables 1e12).5-40 The numerous anatomic de- formed on 11 orbits fixed in formaldehyde. -

THE CENTRAL ARTERY of the RETINA*T H
Br J Ophthalmol: first published as 10.1136/bjo.44.5.280 on 1 May 1960. Downloaded from Brit. J. Ophthal. (1960) 44, 280. THE CENTRAL ARTERY OF THE RETINA*t H. A STUDY OF ITS DISTRIBUHTION AND ANASTOMOSES BY SOHAN SINGH AND RAMJI DASS Department ofAnatomy, Government Medical College, Patiala, India THERE is little unanimity regarding the distribution and anastomoses of the central artery of the retina. According to Frangois and Neetens (1954, 1956) and Fran9ois, Neetens, and Collette (1955), the central artery of the retina is completely devoid of branches, while according to Wolff (1939) and Behr (1935) it is free from branches only in its intraneural course. Magitot (1908), Quain (1909), Beauvieux and Ristitch (1924), Wolff (1940), Bignell (1952), Wybar (1956), and Steele and Blunt (1956) on the other hand demon- strated branches from all parts of its course. Most investigators agree that this artery contributes to the blood supply of the optic nerve, but Francois and others (1954, 1955, 1956) affirm that this is not the case. The presence of a central artery of the optic nerve described by Behr (1935), Wolff (1939, 1954), Francois and others (1954, 1955, 1956), and Wybar (1956), is denied by Beauvieux and Ristitch (1924) and Steele and Blunt (1956). Beauvieux and Ristitch (1924), Kershner (1943), Frangois and others http://bjo.bmj.com/ (1954, 1955, 1956), and Steele and Blunt (1956) say there are no anastomoses between the central retinal and other arteries, but Vail (1948), Wybar (1956), and several other authors have observed and described them. The lamina cribrosa is the only site at which these anastomoses have been studied in any detail, and in this case too, contradictory views have been expressed. -

Acute Hydrops Following Penetrating Keratoplasty in a Keratoconic Patient
Acute Hydrops following PK • Baradaran-Rafiee et al Iranian Journal of Ophthalmology • Volume 20 • Number 4 • 2008 Acute Hydrops following Penetrating Keratoplasty in a Keratoconic Patient Alireza Baradaran-Rafiee, MD1 • Manijeh Mahdavi, MD2 • Sepehr Feizi, MD3 Abstract Purpose: To report a case with history of penetrating keratoplasty (PK) for keratoconus that developed acute hydrops in the recipient and donor cornea Methods: A 46-year-old man, with history of bilateral keratoconus, who had undergone corneal transplantation in his left eye, presented with complaints of sudden visual reduction, photophobia, redness and pain of the left eye. Results: Review of his clinical course, slit-lamp biomicroscopy, laboratory evaluations including confocal microscopy and ultrasound biomicroscopy revealed acute hydrops in the graft. Second corneal transplantation was done for his left eye and pathologic examination confirmed the diagnosis. Conclusion: Acute hydrops can occur after PK in patients with keratoconus. Although this condition is not common, it should be considered as a differential diagnosis of graft rejection. Keywords: Acute Hydrops, Keratoconus, Corneal Transplantation Iranian Journal of Ophthalmology 2008;20(4):49-53 Introduction Keratoconus is a degenerative, One of the complications of advanced non-inflammatory disease of the cornea, with keratoconus is acute hydrops. Affected onset generally at puberty. It is progressive in patients suffer from acute visual loss with pain 20% of cases and can be treated by lamellar and photophobia. On slit-lamp examination, or penetrating keratoplasty (PK). Its incidence conjunctival hyperemia and diffuse corneal in general population is reported to be about edema with intrastromal cystic spaces are 1/2000.1 Changes in corneal collagen visible. -
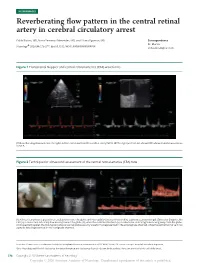
Reverberating Flow Pattern in the Central Retinal Artery in Cerebral
NEUROIMAGES Reverberating flow pattern in the central retinal artery in cerebral circulatory arrest Pablo Blanco, MD, Mar´ıa Fernanda Men´endez, MD, and Liliana Figueroa, MD Correspondence Dr. Blanco Neurology 2020;94:276-277. doi:10.1212/WNL.0000000000008918 ® [email protected] Figure 1 Transcranial Doppler and central retinal arteries (CRA) waveforms (A) Reverberating flow pattern in the right (and left, not shown) middle cerebral artery (MCA). (B) The right (and left, not shown) CRA showed similar waveforms to MCA. Figure 2 Technique for ultrasound assessment of the central retinal arteries (CRA) flow (A) A linear transducer is placed in an axial position over the globe, with the eyelids closed and covered by a generous amount of gel. (B) In color Doppler, the CRA (a) is coded red, indicating flow moving toward the globe (G), while the central retinal vein (v) is coded blue, indicating flow moving away from the globe. (C) In spectral Doppler, the CRA typically shows low resistance velocity waveforms (represented in the anterograde channel), while the central retinal vein has a phasic flow (represented in the retrograde channel). From the “Centro Unico´ Coordinador de Ablacion´ e Implante Provincia de Buenos Aires (CUCAIBA)” Team, “Dr. Emilio Ferreyra” Hospital, Necochea, Argentina. Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article. 276 Copyright © 2020 American Academy of Neurology Copyright © 2020 American Academy of Neurology. Unauthorized reproduction of this article is prohibited. A 49-year-old woman developed signs of brain death after when obtaining flow signals through the cranial bone is not a severe traumatic brain injury. -

The Ophthalmic Artery Ii
Brit. J. Ophthal. (1962) 46, 165. THE OPHTHALMIC ARTERY II. INTRA-ORBITAL COURSE* BY SOHAN SINGH HAYREHt AND RAMJI DASS Government Medical College, Patiala, India Material THIS study was carried out in 61 human orbits obtained from 38 dissection- room cadavers. In 23 cadavers both the orbits were examined, and in the remaining fifteen only one side was studied. With the exception of three cadavers of children aged 4, 11, and 12 years, the specimens were from old persons. Method Neoprene latex was injected in situ, either through the internal carotid artery or through the most proximal part of the ophthalmic artery, after opening the skull and removing the brain. The artery was first irrigated with water. After injection the part was covered with cotton wool soaked in 10 per cent. formalin for from 24 to 48 hours to coagulate the latex. The roof of the orbit was then opened and the ophthalmic artery was carefully studied within the orbit. Observations COURSE For descriptive purposes the intra-orbital course of the ophthalmic artery has been divided into three parts (Singh and Dass, 1960). (1) The first part extends from the point of entrance of the ophthalmic artery into the orbit to the point where the artery bends to become the second part. This part usually runs along the infero-lateral aspect of the optic nerve. (2) The second part crosses over or under the optic nerve running in a medial direction from the infero-lateral to the supero-medial aspect of the nerve. (3) The thirdpart extends from the point at which the second part bends at the supero-medial aspect of the optic nerve to its termination. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division -

Red Spot • Important Is the Time Period from the Event • Therapy Is Reasonable If the Patient Is Treated Within the First 6 Hours
ACUTE VISUAL LOSS KAROLÍNA SKORKOVSKÁ VISUAL LOSS • CENTRAL RETINAL ARTERY OCCLUSION • CENTRAL RETINAL VEIN OCCLUSION • RETINAL DETACHMENT • VITREOUS HAEMORRHAGE • OPTIC NEURITIS • AION VISUAL LOSS - PATIENT´S HISTORY • UNILATERAL / BILATERAL • SUDDEN / SLOWLY PROGRESSIVE • PAIN • COMPLAINTS (FOGGY VISION, FLOATERS, CURTAIN…) • OTHER SYMPTOMS (FEVER, WEIGHT LOSS, REFRACTIVE CHANGE, …) CATARACT • SLOWLY PROGRESSIVE VISUAL LOSS • FOGGY VISION • CHANGE IN REFRACTIVE ERROR (INDEX MYOPIA) • CATARACT VISIBLE ON SLIT LAMP • THERAPY: CATARACT EXTRACTION RETINAL ARTERY OCCLUSION • SUDDEN, UNILATERAL, PAINLESS LOSS OF VISION • VISUAL ACUITY MAY BE „NO LIGHT PERCEPTION“ • BRANCH / CENTRAL RETINAL ARTERY OCCLUSION • RETINAL ISCHEMIA WITH CHERRY RED SPOT • IMPORTANT IS THE TIME PERIOD FROM THE EVENT • THERAPY IS REASONABLE IF THE PATIENT IS TREATED WITHIN THE FIRST 6 HOURS RETINAL ARTERY OCCLUSION - COMPLICATIONS • POOR PROGNOSIS • PERMANENT LOSS OF VISION • OPTIC DISC ATROPHY • NEOVASCULARISATION GLAUCOMA RETINAL ARTERY OCCLUSION - THERAPY • LOWERING OF INTRAOCULAR PRESSURE • RETROBULBAR INJECTION OF VASODILATORS • PARACENTHESIS • SYSTEMIC TROMBOLYSIS (DEPARTMENT OF INTERNAL MEDICINE) • CHECK-UP OF RISK FACTORS OF ISCHEMIA / TROMBOSIS RETINAL VEIN OCCLUSION • SUDDEN, PAINLESS, UNILATERAL VISUAL LOSS • VISUAL ACUITY USUALLY NOT AS MUCH AFFECTED AS IN CRAO • BRANCH / CENTRAL RETINAL VEIN OCCLUSION • OFTEN CARDIOVASCULAR RISK FACTORS (HYPERTENSION, DIABETES, HYPERLIPIDEMIA, ISCHEMIC HEART DISEASE,…) • RETINAL HAEMORHAGES, OPTIC NERVE HEAD OEDEMA, RETINAL -

26. Internal Carotid Artery
GUIDELINES Students’ independent work during preparation to practical lesson Academic discipline HUMAN ANATOMY Topic INTERNAL CAROTID AND SUBCLAVIAN ARTERY ARTERIES 1. The relevance of the topic Pathology of the internal carotid and the subclavian artery influences firstly on the blood supply and functioning of the brain. In the presence of any systemic diseases (atherosclerosis, vascular complications of tuberculosis and syphilis, fibromuscular dysplasia, etc) the lumen of these vessels narrows that causes cerebral ischemia (stroke). So, having knowledge about the anatomy of these vessels is important for determination of the precise localization of the inflammation and further treatment of these diseases. 2. Specific objectives: - define the beginning and demonstrate the course of the internal carotid artery. - determine and demonstrate parts of the internal carotid artery. - determine and demonstrate branches of the internal carotid artery. - determine and demonstrate topography of the left and right subclavian arteries. - determine three parts of subclavian artery, demonstrate branches of each of it and areas, which they carry the blood to. 3. Basic level of knowledge. 1. Demonstrate structural features of cervical vertebrae and chest. 2. Demonstrate the anatomical structures of the external and internal basis of the cranium. 3. Demonstrate muscles of the head, neck, chest, diaphragm and abdomen. 4. Demonstrate parts of the brain. 5. Demonstrate structure of the eye. 6. Demonstrate the location of the internal ear. 7. Demonstrate internal organs of the neck and thoracic cavity. 8. Demonstrate aortic arch and its branches. 4. Task for independent work during preparation to practical classes 4.1. A list of the main terms, parameters, characteristics that need to be learned by student during the preparation for the lesson. -

Anatomy of the Ophthalmic Artery: Embryological Consideration
REVIEW ARTICLE doi: 10.2176/nmc.ra.2015-0324 Neurol Med Chir (Tokyo) 56, 585–591, 2016 Online June 8, 2016 Anatomy of the Ophthalmic Artery: Embryological Consideration Naoki TOMA1 1Department of Neurosurgery, Mie University Graduate School of Medicine, Tsu, Mie, Japan Abstract There are considerable variations in the anatomy of the human ophthalmic artery (OphA), such as anom- alous origins of the OphA and anastomoses between the OphA and the adjacent arteries. These anatomi- cal variations seem to attribute to complex embryology of the OphA. In human embryos and fetuses, primitive dorsal and ventral ophthalmic arteries (PDOphA and PVOphA) form the ocular branches, and the supraorbital division of the stapedial artery forms the orbital branches of the OphA, and then numerous anastomoses between the internal carotid artery (ICA) and the external carotid artery (ECA) systems emerge in connection with the OphA. These developmental processes can produce anatomical variations of the OphA, and we should notice these variations for neurosurgical and neurointerventional procedures. Key words: ophthalmic artery, anatomy, embryology, stapedial artery, primitive maxillary artery Introduction is to elucidate the anatomical variation of the OphA from the embryological viewpoint. The ophthalmic artery (OphA) consists of ocular and orbital branches. The ocular branches contribute to Embryology and Anatomy the blood supply of the optic apparatus, namely, the of the OphA optic nerve and the retina, and the orbital branches supply the optic adnexae, such -

Embryology of the Ophthalmic Artery
Editorial Commentary Interventional Neuroradiology 0(00) 1–2 ! The Author(s) 2019 Embryology of the ophthalmic artery Article reuse guidelines: sagepub.com/journals-permissions DOI: 10.1177/1591019919845511 journals.sagepub.com/home/ine Masaki Komiyama Alternative routes of intra-arterial chemotherapy for misunderstood as dorsal ophthalmic artery).6 The retinoblastoma are based on the embryological and, superficial recurrent ophthalmic artery may run thus, anatomical variations of the ophthalmic artery. through the lateral portion of the SOF, similar to the Although the embryology of the ophthalmic artery is sphenoidal artery or through the lacrimal foramen briefly discussed in this excellent clinical paper,1 I (Hyrtl canal) similar to the lacrimal artery (meningo- would like to comment on this. lacrimal artery) connecting the anterior branch of the The structure of the eye is well conserved among middle meningeal artery to the orbital artery. vertebrates.2 This implies that the vascular structure The embryological origin of the ILT remains specu- of the eye should also be well conserved among verte- lative. It could be the remnant of the primitive maxil- brates, especially among mammals. The basic vascular lary artery of Sabin,7 but this primitive artery dwindles supply of the eye is composed of two sources: one for much earlier than the primitive ventral and dorsal oph- the bulbar structure (retina and choroid structures) and thalmic arteries.5 Thus, the anastomotic branches of the one for the non-bulbar structure (glandular and mus- ILT are mostly composed of the stapedial artery in cular structures),3,4 For an understanding of the origin.3 The primitive maxillary artery is better called embryological origins of these vessels, the bony canal, the primitive pre-mandibular artery in consideration of foramen, and fissure convey many messages to us.