Antidotal Effect of Succimer and Cana 2 EDTA on Workers Exposed To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

Sodium Cellulose Phosphate Sodium Edetate
Sevelamer/Sodium Edetate 1463 excreted by the kidneys. It may be used as a diagnostic is not less than 9.5% and not more than 13.0%, all calculated on supplement should not be given simultaneously with test for lead poisoning but measurement of blood-lead the dried basis. The calcium binding capacity, calculated on the sodium cellulose phosphate. dried basis, is not less than 1.8 mmol per g. concentrations is generally preferred. Sodium cellulose phosphate has also been used for the Sodium calcium edetate is also a chelator of other Adverse Effects and Precautions investigation of calcium absorption. heavy-metal polyvalent ions, including chromium. A Diarrhoea and other gastrointestinal disturbances have Preparations cream containing sodium calcium edetate 10% has been reported. USP 31: Cellulose Sodium Phosphate for Oral Suspension. been used in the treatment of chrome ulcers and skin Sodium cellulose phosphate should not be given to pa- Proprietary Preparations (details are given in Part 3) sensitivity reactions due to contact with heavy metals. tients with primary or secondary hyperparathyroidism, Spain: Anacalcit; USA: Calcibind. Sodium calcium edetate is also used as a pharmaceuti- hypomagnesaemia, hypocalcaemia, bone disease, or cal excipient and as a food additive. enteric hyperoxaluria. It should be used cautiously in pregnant women and children, since they have high Sodium Edetate In the treatment of lead poisoning, sodium calcium calcium requirements. Sodu edetynian. edetate may be given by intramuscular injection or by Patients should be monitored for electrolyte distur- intravenous infusion. The intramuscular route may be Эдетат Натрия bances. Uptake of sodium and phosphate may increase CAS — 17421-79-3 (monosodium edetate). -

Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
1549 The adverse effects of dicobalt edetate are more severe in year-old child: case report and review of literature. Z Kardiol 2005; 94: References. 817-2 3. the absence of cyanide. Therefore, dicobalt edetate should I. Schaumann W, et a!. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur 1 not be given unless cyanide poisoning is confirmed and 30: Administration in renal impairment. In renal impairment, Clin Pharmacol 1986; 527-33. poisoning is severe such as when consciousness is impaired. 2. Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab elimination of antibody-bound digoxin or digitoxin is therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; Oedema. A patient with cyanide toxicity developed delayed1·3 and antibody fragments can be detected in the 28: 483-93. plasma for 2 to 3 weeks after treatment.1 The rebound in 3. Renard C, et al. Pharmacokinetics of dig,'><i<1-sp,eei1ie Fab: effects of severe facial and pulmonary oedema after treatment with decreased renal function and age. 1997; 44: 135-8. dicobalt edetate.1 It has been suggested that when dicobalt free-digoxin concentrations that has been reported after edetate is used, facilities for intubation and resuscitation treatment with digoxin-specific antibody fragments (see P (Jrations should be immediately available. Poisoning, below), occurred much later in patients with r.�p renal impairment than in those with normal renal func Proprietary Preparations (details are given in Volume B) 1. Dodds C, McKnight C. Cyanide toxicity after immersion and the hazards tion. -

WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T (51) International Patent Classification: (74) Agents: CHOTIA, Meenakshi et al; K&S Partners | Intel A 25/12 (2006.01) A61K 8/11 (2006.01) lectual Property Attorneys, 4121/B, 6th Cross, 19A Main, A 25/34 (2006.01) A61K 8/49 (2006.01) HAL II Stage (Extension), Bangalore 560038 (IN). A01N 37/06 (2006.01) A61Q 5/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A O 43/12 (2006.01) A61K 31/44 (2006.01) kind of national protection available): AE, AG, AL, AM, AO 43/40 (2006.01) A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A01N 57/12 (2006.01) A61K 9/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, AOm 59/16 (2006.01) A61K 31/496 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/IB20 14/06 1925 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 3 June 2014 (03.06.2014) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Chelating Drug Therapy: an Update
Open Access Austin Journal of Genetics and Genomic Research Review Article Chelating Drug Therapy: An Update Vijay Kumar1, Ashok Kumar2*, Sandeep Kumar Singh1, Manoj Kumar3, Surendra Kumar2, Dinesh Abstract 4 5 Kumar and Ragni Singh Purpose: To study the clinical effects of metal toxicity and current 1Department of Neurology, SGPGIMS, India recommendations for management, including chelation therapy, are reviewed. 2Department of Medical Genetics, SGPGIMS, India 3Department of Microbiology, SGPGIMS, India Summary: Metals are essential to many biological processes, but excess 4Department of Chemistry, Dr. R.M.L. Avadh University, of it becomes hazardous to life. These are necessary for cell growth, electron India transport chain, several enzymatic activities and response of immune systems. 5Bheem Rao Ambedkar Bihar University, India They also serve as a cofactor for several enzymes. Chelation therapy is used for clinical management of the excess of metal. However, each metal requires *Corresponding author: Ashok Kumar, Department a specific chelation agent. A chelate is a compound form between metal and a of Medical Genetics, Sanjay Gandhi Post Graduate compound that contains two or more potential ligands. A promising Fe chelator Institute of Medical Sciences, Lucknow, India is Desferrioxamine (Desferal). Penicillamine and Trientine are uses for copper Received: March 12, 2015; Accepted: April 24, 2015; chelation. Meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto- Published: April 27, 2015 Propanesulphonate (DMPS) can be used as effective chelator of mercury. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead. Conclusion: Metal toxicity remains a significant public health concern. Elimination of elevated metal ions can be achieved by proper chelation agents. -

Changes in Tissue Gadolinium Biodistribution Measured in an Animal Model Exposed to Four Chelating Agents
Japanese Journal of Radiology (2019) 37:458–465 https://doi.org/10.1007/s11604-019-00835-1 ORIGINAL ARTICLE Changes in tissue gadolinium biodistribution measured in an animal model exposed to four chelating agents Türker Acar1,6 · Egemen Kaya2 · Mustafa Deniz Yoruk3 · Neslihan Duzenli4 · Recep Selim Senturk4 · Cenk Can4 · Lokman Ozturk3 · Canberk Tomruk5 · Yigit Uyanikgil5 · Frank J. Rybicki6 Received: 17 December 2018 / Accepted: 22 March 2019 / Published online: 30 March 2019 © Japan Radiological Society 2019 Abstract Purpose This study investigated the potential to reduce gadolinium levels in rodents after repetitive IV Gadodiamide admin- istration using several chelating agents. Materials and methods The following six groups of rats were studied. Group 1: Control; Group 2: Gadodiamide only; Group 3: Meso-2,3-Dimercaptosuccinic acid (DMSA) + Gadodiamide; Group 4: N-Acetyl-L-cysteine (NAC) + Gadodiamide; Group 5: Coriandrum sativum extract + Gadodiamide; and Group 6: Deferoxamine + Gadodiamide. Brain, kidney, and blood samples were evaluated via inductively coupled plasma mass spectrometry. The brain was also evaluated histologically. Results Kidney gadolinium levels in Groups 4 and 5 were approximately double that of Group 2 (p = 0.033 for each). There was almost no calcifcation in rat hippocampus for Group 4 rodents when compared with Groups 2, 3, 5 and 6. Conclusion Our preliminary study shows that excretion to the kidney has a higher propensity in NAC and Coriandrum sati- vum groups. It may be possible to change the distribution of gadolinium by administrating several agents. NAC may lower Gadodiamide-induced mineralization in rat hippocampus. Keywords Gadolinium deposition · Dimercaptosuccinic acid · N-Acetyl-L-cysteine · Coriandrum sativum · Deferoxamine Introduction However, the safety profle of gadolinium-based agents [1] was questioned after the discovery of nephrogenic sys- Gadolinium-based contrast agents (GBCA) have been safely temic fbrosis [2], a scleroderma-like disease characterized used in diagnostic radiology since the 1980s. -
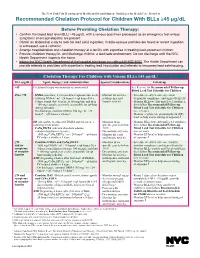
Recommended Chelation Protocol for Children with Blls ≥45 Μg/Dl
The New York City Department of Health and Mental Hygiene Guidelines for Health Care Providers Recommended Chelation Protocol for Children With BLLs ≥45 μg/dL Before Providing Chelation Therapy: • Confirm the blood lead level (BLL) ≥45 μg/dL with a venous specimen processed as an emergency test unless symptoms of encephalopathy are present. • Obtain an abdominal x-ray to look for lead solid ingestion; if radio-opaque particles are found or recent ingestion is witnessed, use a cathartic. • Arrange hospitalization and chelation therapy at a facility with expertise in treating lead-poisoned children. • Provide chelation therapy in, and discharge child to, a lead-safe environment. Do not discharge until the NYC Health Department inspects the home. • Inform the NYC Health Department of the hospital admission by calling 646-632-6002. The Health Department can provide referrals to providers with expertise in treating lead intoxication and referrals to temporary lead-safe housing. Chelation Therapy For Children with Venous BLLs ≥45 μg/dL1 BLL (μg/dL) Agent, Dosage,* and Administration Special Considerations Follow-up <45 Chelation therapy not routinely recommended See Reverse for Recommended Follow-up Blood Lead Test Schedule for Children 45 to <70 • DMSA (succimer, 2,3-meso-dimercaptosuccinic acid) • Monitor for anemia, • Schedule weekly health care visits • 1050 mg DMSA / m2 / 24 hours* ÷ q8 hours PO x neutropenia, and to monitor compliance and signs of toxicity. 5 days; round dose to nearest 100 mg/day, and then hepatic toxicity. • Monitor BLLs weekly until level stabilizes, ÷ 100-mg capsules as evenly as possible for q8-hour then follow Recommended Follow-up dosing schedule. -

Effect of Chromium(VI) on Serum Iron and Removal of Its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats
American Journal of Pharmacology and Toxicology 8 (4): 164-169, 2013 ISSN: 1557-4962 ©2013 Science Publication doi:10.3844/ajptsp.2013.164.169 Published Online 8 (4) 2013 (http://www.thescipub.com/ajpt.toc) Effect of Chromium(VI) on Serum Iron and Removal of its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats 1S. Jamil A. Fatemi, 1Marzieh Iranmanesh and 2Faezeh Dahooee Balooch 1Department of Chemistry, Faculty of Sciences, Islamic Azad University, Kerman Branch, Kerman, Iran 2Department of Chemistry, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran Received 2013-09-26, Revised 2013-10-21; Accepted 2013-10-30 ABSTRACT The present research is aimed to characterize the potential efficiency of two chelators after chromium(VI) administration for 60 days following two doses of 15 and 30 mg kg −1 chromium(VI) per body weight daily to male rats. However, the hypothesis that the two chelators might be more efficient as combined therapy than as single therapy in removing chromium(VI) from bood serum was considered. In this way, two known chelators deferasirox and deferiprone were chosen and tested in the acute rat model. Two chelators were given orally as a single or combined therapy for a period of one week. Chromium(VI) and iron concentrations in blood were determined by flame atomic absorption spectroscopy method. Chromium is one of the most widely used industrial metals. Several million workers worldwide are estimated to be exposed to chromium compounds in an array of industries. Chromium(VI) is more readily absorbed by both inhalation and oral routes. Ingestion of large amounts of chromium(VI) can lead to severe respiratory, cardiovascular, gastrointestinal, hepatic and renal damage and potentially death. -

AX Pharmaceutical Corp Product List
AX Pharmaceutical Corp Product List A 4-Aminopyridine Acetazolamine ACTH 1-24 Acyclovir Sodium Adenosine 5 Monophosphate Adenosine 5 Tri-Phosphate Disodium Salt Albendazole Alendronate Sodium USP Alternogest Aminopentamide Sulfate Aminophylline Amlodipine Besylate Amoxicillin/Clavulanate Potassium 4:1 Amoxicillin Trihydrate Amphotericin B Ampicillin Anastrozole Aripepazole Aripiprazole Atipamezole HCl Atovaquone USP Atropine Sulfate Monohydrate Avanafil Azelastine HCl B Baclofen Benazepril HCl Betahistine Dihydrochloride Betaine Hydrochloride Betamethasone Acetate Betamethasone Dipropionate Betamethasone Sodium Phosphate Betaxolol Bexarotene Bicalutamide Bimatoprost Bisacodyl Bismuth Subcarbonate Bismuth Subsalicylate Bleomycin A5 Hydrochloride Bleomycin Sulfate Bretylium Tosylate Brimonidine Brinzolamide Bromhexine Bromocriptine Mesylate Brompheniramine Maleate Budesonide Bumetanide Bupivacaine Base Bupivacaine Hydrochloride Buprenorphine Bupropion HCl Buspirone Hydrochloride Busulfan Butaphosphan Butylated Hydroxyanisole C Cabergoline Calamine Calcium Glycerophosphate Calcium Levulinate Dihydrate Capsaicin Captopril Carbamazepine Carbazochrome Carbenoxolone Carbetocin Acetate Carbidopa Carbocisteine Carboplatin Carmustine Carprofen Carvedilol Cefadroxil Hemihydrate Cefadroxil Monohydrate Cefazolin Cefazolin Sodium Cefdinir Cefotaxime Cefotetan Disodium Cefpodoxime Cefpodoxime Proxetil Ceftazidime Ceftiofur Free Acid Ceftiofur Sodium Ceftriaxone Cefuroxime (Ceftin) Celecoxib Cephalexin Base Cephalexin Monohydrate Cesium Chloride Cetirizine -

The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island Poison Potential Antidote Acetaminophen n-Acetylcysteine [Mucomyst®] Anticholinergics Physostigmine [Antilirium®] Benzodiazepines Flumazenil [Romazicon®] Beta-adrenergic blockers Glucagon Botulinum toxin Trivalent ABE botulinum antitoxin Calcium chloride or calcium gluconate Calcium channel blockers Hyperinsulinemia-euglycemia (HIE) therapy Atropine Carbamates Pralidoxime (2-PAM) [Protopam®] Carbon monoxide Oxygen; Hyperbaric oxygen (HBO) Clonidine Naloxone [Narcan®] Cyanide Cyanide Kit (Amyl/sodium nitrite, sodium thiosulfate) Digoxin (Cardiac glycosides) Digoxin Immune FAB Ovine [Digibind®, Digifab®] Epi Pen (Epinephrine SQ) Phentolamine [Regitine®] Ethanol Ethylene glycol 4-Methylpyrazole (Fomepizole) [Antizol®] Fluoride Calcium chloride or calcium gluconate Heparins Protamine Hydrofluoric acid Calcium chloride or calcium gluconate Hydrogen sulfide Oxygen; Hyperbaric oxygen (HBO); Sodium nitrite Iodine Starch Isoniazid Pyridoxine (Vitamin B6 ) METALS Dimercaprol [BAL] Arsenic Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Bismuth Dimercaprol [BAL] Copper D-Penicillamine [Cuprimine®] Gold Dimercaprol [BAL] Iron Deferoxamine [Desferal®] Dimercaprol [BAL] Edetate calcium disodium (Calcium EDTA) Lead Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] D-Penicillamine [Cuprimine®] Dimercaprol [BAL] Mercury Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Ethanol Methanol 4-Methylpyrazole (Fomepizole) [Antizol®] Methemoglobinemic agents Methylene -

Poison Control Antidote Poster
Antidote Chart (The suggested minimum stocking level is a combination of factors; anticipation of the highest total dose of a drug generally given during a 24 hour period to a 70 kg adult.) General Decontamination Uses Dose Comments Activated Charcoal (without sorbitol) Most ingestions occurring within one hour Adults: 25–100 grams, Children: 1 g/kg May be given in multiple doses depending on ingestant to enhance elimination Activated Charcoal (with sorbitol) May be used as first AC given to patient if presents within Adults: 25–50 grams Should not be used for multiple dose activated charcoal regimens one hour of ingestion Children: Not generally recommended Whole Bowel Irrigation Drugs not bound by charcoal, sustained Adults: 500–2000 ml/hour Nasogastric tube should be used to maintain amount given (Polyethylene Glycol) release formulations, and body stuffers Children: 25 ml/kg/hour Syrup of Ipecac No longer recommended No longer recommended No longer recommended Poisoning Antidote Quantity to Stock* Comments Acetaminophen N-ACETYLYCYSTEINE 10–20% 600 ml (20% Mucomyst®) Because vomiting of the oral NAC is common, the facility should maintain a repeat dose (Mucomyst®) or 1200 ml (10% Mucomyst®) for each patient for initial dosing if continuation of treatment at facility is not anticipated. ACETADOTE® The IV Acetadote® was just approved in February 2004. Call the MAPCC for dosing, ACETYLCYSTEINE Injection for IV use 4 (30 ml) vials of 20% solution precautions and contraindications. Both the oral and IV forms of acetylcysteine should be administered within 8 hours for maximal protection against hepatic injury. Anticholinergic Poisoning PHYSOSTIGMINE (Antilirium®) 2–4 mg* Not generally recommended for children. -

Heavy Metal Chelation and Testing
Heavy Metal Chelation and Testing Background Information Chelation is the use of a chemical substance to bind molecules, such as metals or minerals, and hold them tightly so they can be removed from the body. The main heavy metal found on testing in the general population is mercury. Fish consumption is directly associated with increased methylmercury levels1, while mercury in dental amalgams is probably the major source of inorganic mercury exposure in humans and studies have shown a direct correlation between the number of amalgam fillings and mercury concentration in blood and urine2. The impact of chronic, low level mercury exposure is now known to adversely impact numerous cellular and organ system processes3. Ionic mercury is antigenic and may contribute significantly to autoimmune processes4, 5. Mercury is also immunotoxic and it may result in immune suppression and allergy6,7,8,9. Research has also demonstrated that multiple strains of antibiotic resistant bacteria develop rapidly in the gut and oral cavity of both humans as well as non-human primates following the placement of amalgam fillings10. Liver glutathione production can be markedly inhibited when mercury accumulates within hepatocytes (liver cells), causing mercury and numerous other toxic substances to accumulate throughout the body11. Mercury may also promote atherosclerosis and hence increase the risk of myocardial infarction12. Methylmercury is considered the most toxic form of mercury and targets the central nervous system whereas inorganic mercury favours accumulation in the kidneys. Research has demonstrated that DMPS13 (2,3-dimercapto-1-propane sulfonic acid) is a good chelating agent for inorganic mercury and DMSA (2,3- dimercaptosuccinic acid ) is considered the most efficient chelator of methylmercury14 and lead15 16.