Isolation and Enumeration of Fungi and Determination Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
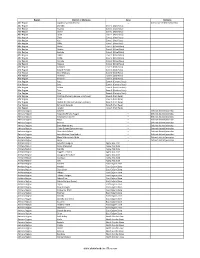
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Honey Bee Floras Along the Agro-Ecology, Jimma Zone, Southwest Ethiopia
ISSN 2664-4169 (Print) & ISSN 2664-7923 (Online) South Asian Research Journal of Biology and Applied Biosciences Abbreviated Key Title: South Asian Res J Bio Appl Biosci | Volume-3 | Issue-4 | July-Aug -2021 | DOI: 10.36346/sarjbab.2021.v03i04.001 Original Research Article Honey Bee Floras along the Agro-Ecology, Jimma Zone, Southwest Ethiopia Abera Hailu Degaga1*, Minyahel Tilahun1 1College of Agriculture and Natural Resource, Wolkite University, P.O.Box 07, Wolkite, Ethiopia *Corresponding Author Abera Hailu Degaga Email: [email protected] Article History Received: 29.06.2021 Accepted: 03.08.2021 Published: 08.08.2021 Abstract: Ethiopia is home to diverse plant species that provide nectar and pollen as bees forage. In this study, honey bee forages were assessed in three different agro-ecology districts, Jimma Zone, Southwest, Ethiopia. Random sampling techniques was used to collect the data, ninety beekeepers were interviewed using structured questioner. Key informants were interviewed with in all study areas. ANOVA of GLM and Regression were done using Minitab statistical software. Accordingly 42 honey bee’ forages; 28 trees, 6 shrubs and 8 herbs were mentioned by respondents which belong to 22 families, Fabaceae and Asteraceae were the first and second dominant family respectively. Natural forest trees, cultivated crops and fruits were identified as bee forage. Beekeepers experience and their knowledge on seasonal availability of bee forage and honey bee poisonous plants found in their locality were also assessed. In the study area traditional forest beekeeping system is practiced to produce honey. Different bee forages bear flower at different months and visited by honey bees for different number of days. -

On Farm Production Systems Characterization of Indigenous Cattle in Bako Tibe and Gobu Sayo Districts of Oromia Region, Ethiopia
Journal of Biology, Agriculture and Healthcare www.iiste.org ISSN 2224-3208 (Paper) ISSN 2225-093X (Online) Vol.6, No.22, 2016 On Farm Production Systems Characterization of Indigenous Cattle in Bako Tibe and Gobu Sayo Districts of Oromia Region, Ethiopia Dereje Bekele 1 Kefelegn Kebede 2 A.K. Banarjee 2 1.Oromia Agricultural research Institute, Bako Agricultural Research center, Bako, Ethiopia 2.Haramaya University, School of Animal and Range Sciences, Haramaya, Ethiopia Abstract The study was conducted in Bako Tibe and Gobu Sayo districts of Oromia Regional State, Ethiopia, from October 2014 to January 2015 with the objective to undertake on-farm production system characterization of indigenous cattle breed (Horro) in the study area. Field studies and collection of data were carried out through semi-structured questionnaire, focus group discussions, key informants and secondary data collection from different sources. A total of 120 households (60 from each district) were randomly selected for semi structured questionnaire interview. SAS and SPSS software were used to analyze the data. The study result revealed that overall cattle herd size was 9.67±3.34 heads per household and was not significantly different (p<0.05) between districts. The main purposes of keeping Horro cattle in both locations were draught power, milk production, income, manure and threshing of crop. The age at first service (AFS) of male Horro cattle was 3.47±0.39 years. The age at first mating (AFM) and age at first calving (AFC) of female cattle were 3.73±0.51 and 4.98±0.68 years respectively. The calving interval (CI) of Horro cow was estimated to be 1.88±0.49 years and showed no significant difference between locations. -

Determinants of Dairy Product Market Participation of the Rural Households
ness & Fi si na u n c B Gemeda et al, J Bus Fin Aff 2018, 7:4 i f a o l l A a Journal of f DOI: 10.4172/2167-0234.1000362 f n a r i r u s o J ISSN: 2167-0234 Business & Financial Affairs Research Article Open Access Determinants of Dairy Product Market Participation of the Rural Households’ The Case of Adaberga District in West Shewa Zone of Oromia National Regional State, Ethiopia Dirriba Idahe Gemeda1, Fikiru Temesgen Geleta2 and Solomon Amsalu Gesese3 1Department of Agricultural Economics, College of Agriculture and Veterinary Sciences, Ambo University, Ethiopia 2Department of Agribusiness and Value Chain Management, College of Agriculture and Veterinary Sciences, Ambo University, Ethiopia Abstract Ethiopia is believed to have the largest Livestock population in Africa. Dairy has been identified as a priority area for the Ethiopian government, which aims to increase Ethiopian milk production at an average annual growth rate of 15.5% during the GTP II period (2015-2020), from 5,304 million litters to 9,418 million litters. This study was carried out to assess determinants of dairy product market participation of the rural households in the case of Adaberga district in West Shewa zone of Oromia national regional state, Ethiopia. The study took a random sample of 120 dairy producer households by using multi-stage sampling procedure and employing a probability proportional to sample size sampling technique. For the individual producer, the decision to participate or not to participate in dairy production was formulated as binary choice probit model to identify factors that determine dairy product market participation. -

Farmers' Willingness to Pay for Improved Forage Seed in LIVES
ii Farmers’ Willingness to Pay for Improved Forage Seed in LIVES Districts of West Shewa Zone, Ethiopia A Thesis Submitted to the College of Agriculture and Environmental Sciences, the School of Agricultural Economics and Agribusiness, School of Graduate Studies HARAMYA UNIVERSITY In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN AGRICULTURE (AGRICULTURAL ECONOMICS) Lemi Gonfa June, 2015 Haramaya University, Haramaya iii APPROVAL SHEET SCHOOL OF GRADUATE STUDIES HARAMAYA UNIVERSITY I hereby certify that I have read and evaluated this thesis entitled Farmers’ Willingness to Pay for Improved Forage Seed in LIVES districts of West Shewa Zone, Ethiopia prepared under my guidance by Lemi Gonfa. I recommend that it can be submitted as fulfilling the Thesis requirement. Lemma Zemedu (PhD) _________________ _______________ Major Advisor Signature Date Berhanu Geberemedih (PhD) _________________ _______________ Co-advisor Signature Date As member of the Board of Examiners of the MSc Thesis Open Defense Examination , I certify that I have read, evaluated the Thesis prepared by Lemi Gonfa and examined the candidate. I recommended that the Thesis be accepted as fulfilling the Thesis requirement for the Degree of Master of Science in Agriculture (Agricultural Economics). Mengistu Ketema (PhD ) _________________ _______________ Chairperson Signature Date Jema Haji (PhD) _________________ _______________ Internal Examiner Signature Date Endrias Geta (PhD) _________________ _______________ External Examiner Signature Date Final approval and acceptance of the Thesis is contingent upon the submission of its final copy to the Council of Graduate Studies (CGS) through the candidate’s department or school of graduate committee (DGC or SGC). iv DEDICATION This thesis work is dedicated to my wife, Atsede Molla for taking care of my lovely son Naol Lemi and little princess Bersabeh Lemi during my absence and for nursed me with affection and love throughout my work. -

Globalization: Global Politics and Culture (Msc)
LAND GRAB IN ETHIOPIA: THE CASE OF KARUTURI AGRO PRODUCTS PLC IN BAKO TIBE, OROMIYA Dejene Nemomsa Aga Supervisor: Professor Lund Ragnhild Master Thesis Faculty of Social Sciences and Technology Management Department of Geography Globalization: Global Politics and Culture (MSc). May 2014, Trondheim, Norway Globalization: Global Politics and Culture (M.Sc) Declaration I, the undersigned, declare that this thesis is my original work and all materials used as a source are duly acknowledged. Name:........................ Dejene Nemomsa Aga Date:……………........... 23 May, 2014 Dejene Nemomsa Aga Page i Globalization: Global Politics and Culture (M.Sc) Dedication I dedicate my master thesis work to anti-land grabbing protesters of Oromo Students and People, who were recently killed while protesting the implementation of ‘Integrated Development Master Plan of the Capital City of the country, Finfinne’, which planned to displace more than one million indigenous Oromo People from their ancestral land. 23 May, 2014 ii Dejene Nemomsa Aga Globalization: Global Politics and Culture (M.Sc) Acknowledgements Firsts, I would like to thank the almighty God. Next, my special thanks go to my advisor Professor Ragnhild Lund, for her guidance and detailed constructive comments that strengthened the quality of this thesis. Professor’s countless hours of reflecting, reading, encouraging, and patience throughout the entire process of the research is unforgettable. I would like to thank Norwegian University of Science and Technology for accepting me as a Quota Scheme Student to exchange knowledge with students who came from across the globe and Norwegian State Educational Loan Fund for covering all my financial expenses during my stay. My special thanks go to department of Geography, and Globalization: Global Politics and Culture program coordinators, Anette Knutsen for her regular meetings and advice in the research processes. -

ETHIOPIA Selfhelpafrica.Org 2020-21 1 2020-21 Alemnesh Tereda, 28, and Marsenesh Lenina, 29, Injaffo Multi Barley Coop, Gumer
ETHIOPIA selfhelpafrica.org 2020-21 1 2020-21 Alemnesh Tereda, 28, and Marsenesh Lenina, 29, Injaffo Multi barley Coop, Gumer caling up agricultural production, improving nutrition Last year, the organisation was involved in implementing security, developing new enterprise and market close to a dozen development projects, all of which Sopportunities for farmers, strengthening community- are being undertaken in collaboration with local and/or based seed production and building climate resilience, are international partners. all key areas of Self Help Africa’s work in Ethiopia. ETHIOPIA PROJECT KEY Scaling up RuSACCOs Strengthening & Scaling up of rehabilitaion of degraded lands and enhancement of livelihoods in Lake Ziway catchment ERITREA Feed the Future Gondar Dairy for Development Stronger Together: Linking Primary Seed and Seep Cooperative Union Addis Ababa Climate-Smart Agriculture SOMALILAND Capacity Building of Farmer Butajira Training Centers Unleashing the productive ETHIOPIA capacity of poor people through Graduation Approach in Ethiopia Integrated Community Development SOMALIA Livelihood Enhancement: Working Inclusively for Transformation KENYA 2 Implementing Programme Programme Donor Total Budget Time Frame Partner Area Climate-Smart Irish Aid € 806,695 2015 SOS Sahel, SNNP region 01 Agriculture (CSA) Farm Africa, 2019 Vita MF: Scaling Up Irish League of € 420,000 2020 Zonal Departments of N/Shewa Zone of 02 Rural Savings and Credit international Finance & Economic Amhara, N/Shewa Credit Cooperatives Development 2022 Cooperation -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

Heading with Word in Woodblock
Oromia Region, Area brief Regional Overview Oromia (sometimes spelled Oromiya, in the Oromo language) is one of the nine regions of Ethiopia. The 2007 census reported its population at over 28 million, making it the largest state in terms of both population and area. Oromia shares a boundary with every Region of Ethiopia except for the Tigray Region. With an estimated area of 353,006.81 square kilometers, this region has an estimated population density of 76.93 people per square kilometer. The region includes the former major Ethiopian provinces Arsi, Bale, Hararghe, Illubabor, Kaffa, Shewa, Sidamo, and Welega provinces. Its current capital is officially Addis Ababa (Oromo: Finfinne). Administratively, Adama serves as a center for the regional state. Other important cities and towns include Adama, Ambo, Asella, Bishoftu, Dembidolo, Fiche, Gimbi, Goba, Jimma, Metu, Negele Boran, Nekemte, Shashamane and Waliso. The Regional infant mortality rate is 76 infant deaths per 1,000 live births, similar to the nationwide average of 77; at least half of these deaths occurr in the infants’ first month of life. Low latrine coverage and little awareness of basic hygiene practices across many parts of the region are having a significant impact on the health and wellbeing of children and their families. In view of the above Save the Children in collaboration with the government and other key allies’ works to achieve MDG 4 and 5 by reducing maternal, newborn and child deaths. As part of our EVERYONE campaign we work to raise awareness in communities about safe delivery and child caring practices. We also work with relevant partners to improve the WASH services and practices at household and facility level. -

Seroprevalence and Associated Risk Factors of Foot and Mouth Disease in Cattle in West Shewa Zone, Ethiopia
Hindawi Veterinary Medicine International Volume 2020, Article ID 6821809, 6 pages https://doi.org/10.1155/2020/6821809 Research Article Seroprevalence and Associated Risk Factors of Foot and Mouth Disease in Cattle in West Shewa Zone, Ethiopia Beyan Ahmed,1 Lencho Megersa ,2 Getachew Mulatu ,2 Mohammed Siraj,2 and Gelma Boneya2 1Department of Veterinary Science, College of Agriculture and Veterinary Science, Ambo University, P.O. Box 19, Ambo, Ethiopia 2Department of Veterinary Laboratory Technology, College of Agriculture and Veterinary Sciences, Ambo University, P.O. Box 19, Ambo, Ethiopia Correspondence should be addressed to Lencho Megersa; [email protected] Received 7 November 2019; Revised 19 February 2020; Accepted 7 March 2020; Published 31 March 2020 Academic Editor: Annamaria Pratelli Copyright © 2020 Beyan Ahmed et al. (is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Foot and mouth disease (FMD) is a highly contagious viral disease of cloven-hoofed animals and one of the endemic diseases in Ethiopia. (e study was aimed to estimate the seroprevalence and to assess associated risk factors of foot and mouth disease seroprevalence in West Shewa Zone. A total of 384 sera samples were collected from randomly selected cattle and tested using ELISA for antibodies against nonstructural proteins of foot and mouth disease viruses based on IDEXX FMD Multispecies Ab Test (IDEXX Laboratories Inc, USA). (e seroprevalence of foot and mouth disease in West Shewa Zone was found to be 40.4% (95% CI: 35.46–45.27) at an animal and 74.7% (95% CI: 65.58–83.85) at the herd level. -

Water Rights and the Processes of Negotiations Among Irrigators in West Shewa Zone: the Case of Indris Scheme in Toke Kutaye District
Water Rights and the Processes of Negotiations among Irrigators in West Shewa Zone: The Case of Indris Scheme in Toke Kutaye District Tesfaye Zeleke Axum University [email protected] Abstract Conflicts in connection to irrigation water use and rights that have escalated over Though water rights are at the core of exploiting water resources for irrigation Years have been attributed to the decline in purposes, trivial concerns were offered to the the volume of water resources, institutional case of Indris irrigation scheme in Toke failures to address the causes adequately, Kutaye district in West Shewa. The historical week observance on governing water right background and development of the scheme rules and increasing demand of users. As a has been presented in a contentious manner. result, negotiation processes aiming to settle The augmenting number of competitors too disputes were repeatedly initiated either by paved the way for conflicts that recurrently users, committee members (elders) or courts. erupt out and inevitably lead to a succession The procedures pursed to narrow competing of negotiation processes. With the inception interests around the scheme confirmed the of such missing gaps, this research aimed to pragmatic applicability of the central scrutinize water rights and the processes of arguments of both cyclical and negotiations among irrigators along Indris developmental models of negotiation modern scheme, in Toke Kutaye district. To processes discussed comprehensively by maintain this objective, qualitative research Gulliver. methods were predominantly utilized as the main data generating tools in the field. Thus, in the face of increasing demands on a declining water resource, the findings of this The findings of the research depicted that research revealed out that concerned Indris scheme marked three significant individuals or relevant institutions need to phases in its historical development. -

Prevalence, Risk Factors and Antimicrobial Suscep- Tibility Profile of Salmonella Isolated from Dogs of Ambo, Bako and Gojo Towns of West Shoa, Ethiopia
Zewdu et al., Ethiop. Vet. J., 2019, 23 (1), 59-77 DOI https://dx.doi.org/10.4314/evj.v23i1.5 Ethiopian Veterinary Journal Prevalence, risk factors and antimicrobial suscep- tibility profile of Salmonella isolated from dogs of Ambo, Bako and Gojo towns of West Shoa, Ethiopia Endrias Zewdu Gebremedhin*1, Sisay Miheretu2, Lencho Megersa3, Edilu Jorga Sarba1, Getachew Kebebew1 and Solomon Shiferaw3 1Department of Veterinary Sciences, College of College of Agriculture and Veterinary Science, Ambo University, P.O.Box 19, Ambo, Ethiopia. 2Department of Animal Production and Technology, College of Agricultural Sciences, Welkite University, P.O.Box 07, Welkite,Guragie Zone, SNNPR, Ethiopia. 3Department of Veterinary Laboratory Technology, College of Agriculture and Veterinary Science, Ambo University, P.O.Box 19, Ambo, Ethiopia *Corresponding author: Endrias Zewdu Gebremedhin [email protected], Abstract Salmonella is the most known zoonotic bacterial agent, which produces sal- monellosis in animals as wells as in humans. The objectives of this study were to estimate the prevalence, to determine antimicrobial susceptibility and to assess risk factors associated with Salmonella shedding in dogs in selected towns of West Shoa Zone, Oromia, Ethiopia. Using a cross-sectional design, a total of 438 rectal swab samples were collected from randomly selected dogs for isolation and identification of Salmonella using standard procedures. A ques- tionnaire survey was also administered. The results showed that 48 (11.0%, 95%, [CI]: 8.2% - 14.3 %) dogs were positive for Salmonella.The occurrenceof Salmonella was 10.9% (26/238), 11.6% (15/129) and 9.9% (7/71) in Ambo, Bako and Gojo towns respectively.