Cortical Complexity in Cetacean Brains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Non-Canonical Feedforward Pathway for Computing Odor Identity
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.317248; this version posted September 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A non-canonical feedforward pathway for computing odor identity Honggoo Chae1♯, Arkarup Banerjee1,2,3♯ & Dinu F. Albeanu1,2* 1 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 2 Cold Spring Harbor Laboratory School for Biological Sciences, Cold Spring Harbor, NY 3 current address - New York University Medical Center, New York, NY ♯ equal contribution * Correspondence: [email protected] Short title: Cell-type specific decoding of odor identity and intensity in the olfactory bulb Key words: mitral and tufted cells, piriform cortex, anterior olfactory nucleus, cortical feedback, concentration invariant odor identity decoding, two photon calcium imaging, PCA, dPCA, linear and non-linear decoders bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.317248; this version posted September 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Sensory systems rely on statistical regularities in the experienced inputs to either group disparate stimuli, or parse them into separate categories1,2. While considerable progress has been made in understanding invariant object recognition in the visual system3–5, how this is implemented by olfactory neural circuits remains an open question6–10. The current leading model states that odor identity is primarily computed in the piriform cortex, drawing from mitral cell (MC) input6–9,11. -
![Abnormalities of Grey and White Matter [11C]Flumazenil Binding In](https://docslib.b-cdn.net/cover/7913/abnormalities-of-grey-and-white-matter-11c-flumazenil-binding-in-217913.webp)
Abnormalities of Grey and White Matter [11C]Flumazenil Binding In
Brain (2002), 125, 2257±2271 Abnormalities of grey and white matter [11C]¯umazenil binding in temporal lobe epilepsy with normal MRI A. Hammers,1,2,3 M. J. Koepp,1,2,3 R. Hurlemann,2 M. Thom,2 M. P. Richardson,1,2,3 D. J. Brooks1 and J. S. Duncan1,2,3 1MRC Clinical Sciences Centre and Division of Correspondence to: Professor John S. Duncan, MA, DM, Neuroscience, Faculty of Medicine, Imperial College, FRCP, National Society for Epilepsy and Institute of 2Department of Clinical and Experimental Epilepsy, Neurology, 33 Queen Square, London WC1N 3BG, UK Institute of Neurology, University College London, London E-mail: [email protected] and 3National Society for Epilepsy MRI Unit, Chalfont St Peter, UK Summary In 20% of potential surgical candidates with refrac- the 16 patients with abnormalities, ®ndings were con- tory epilepsy, current optimal MRI does not identify cordant with EEG and clinical data, enabling further the cause. GABA is the principal inhibitory neuro- presurgical evaluation. Group ®ndings were: (i) transmitter in the brain, and GABAA receptors are decreased FMZ-Vd in the ipsilateral (Z = 3.01) and expressed by most neurones. [11C]Flumazenil (FMZ) contralateral (Z = 2.56) hippocampus; (ii) increased PET images the majority of GABAA receptor sub- FMZ-Vd in the ipsilateral (Z = 3.71) and contralat- types. We investigated abnormalities of FMZ binding eral TLWM (two clusters, Z = 3.11 and 2.79); and in grey and white matter in 18 patients with refrac- (iii) increased FMZ-Vd in the ipsilateral frontal lobe tory temporal lobe epilepsy (TLE) and normal quan- white matter between the superior and medial frontal titative MRI. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

The Connexions of the Amygdala
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.2.137 on 1 April 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 137 The connexions of the amygdala W. M. COWAN, G. RAISMAN, AND T. P. S. POWELL From the Department of Human Anatomy, University of Oxford The amygdaloid nuclei have been the subject of con- to what is known of the efferent connexions of the siderable interest in recent years and have been amygdala. studied with a variety of experimental techniques (cf. Gloor, 1960). From the anatomical point of view MATERIAL AND METHODS attention has been paid mainly to the efferent connexions of these nuclei (Adey and Meyer, 1952; The brains of 26 rats in which a variety of stereotactic or Lammers and Lohman, 1957; Hall, 1960; Nauta, surgical lesions had been placed in the diencephalon and and it is now that there basal forebrain areas were used in this study. Following 1961), generally accepted survival periods of five to seven days the animals were are two main efferent pathways from the amygdala, perfused with 10 % formol-saline and after further the well-known stria terminalis and a more diffuse fixation the brains were either embedded in paraffin wax ventral pathway, a component of the longitudinal or sectioned on a freezing microtome. All the brains were association bundle of the amygdala. It has not cut in the coronal plane, and from each a regularly spaced generally been recognized, however, that in studying series was stained, the paraffin sections according to the Protected by copyright. the efferent connexions of the amygdala it is essential original Nauta and Gygax (1951) technique and the frozen first to exclude a contribution to these pathways sections with the conventional Nauta (1957) method. -
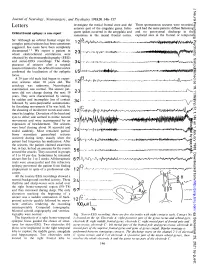
Letters Anterior Part of the Cingulate Gyrus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.1.146 on 1 January 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:146-157 investigate the mesial frontal zone and the Three spontaneous seizures were recorded, Letters anterior part of the cingulate gyrus. Infre- each had the same pattern: diffuse flattening quent spikes occurred in the amygdala and and no in the Orbital frontal epilepsy: a case report paroxysmal discharge sometimes in the mesial frontal cortex. explored sites in the frontal or temporal Sir: Although an orbital frontal origin for A complex partial seizures has been sometimes 12 suggested, few cases have been completely documented.1 2 We report a patient in 23 whom electroclinical correlations were obtained by electroencephalography (EEG) and stereo-EEG recordings. The disap- 3.4 I-w VI,-#a+r - pearance of seizures after a surgical resection limited to the orbital frontal cortex confirmed the localisation of the epileptic 4.5 o I\A-.ViJV "fJ4 WOO focus. A 29 year old male had begun to experi- B ence seizures when 10 years old. The aetiology was unknown. Neurological examination was normal. The seizure pat- terns did not change during the next 19 23 ., years. They were characterised by staring, r by sudden and incomplete loss of contact -- - -. , A- followed by semi-purposeful automatisms, 3-4 v by thrashing movements if he was held, by the shouting of incoherent words and some- times by laughter. Deviation of the head and eyes to either side seemed to mimic natural Protected by copyright. -

Corticostriatal Interactions During Learning, Memory Processing, and Decision Making
The Journal of Neuroscience, October 14, 2009 • 29(41):12831–12838 • 12831 Mini-Symposium Corticostriatal Interactions during Learning, Memory Processing, and Decision Making Cyriel M. A. Pennartz,1 Joshua D. Berke,2,3 Ann M. Graybiel,4,5 Rutsuko Ito,6 Carien S. Lansink,1 Matthijs van der Meer,7 A. David Redish,7 Kyle S. Smith,4,5 and Pieter Voorn8 1University of Amsterdam, Swammerdam Institute for Life Sciences Center for Neuroscience, 1098 XH Amsterdam, The Netherlands, 2Department of Psychology and 3Neuroscience Program, University of Michigan, Ann Arbor, Michigan 48109, 4Department of Brain and Cognitive Sciences and 5McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, 6Department of Experimental Psychology, University of Oxford, Oxford OX1 3UD, United Kingdom, 7Department of Neuroscience, University of Minnesota, Minneapolis, Minnesota 55455, and 8Department of Anatomy, Research Institute Neurosciences, Vrije Universiteit University Medical Center, 1007 MB Amsterdam, The Netherlands This mini-symposium aims to integrate recent insights from anatomy, behavior, and neurophysiology, highlighting the anatom- ical organization, behavioral significance, and information-processing mechanisms of corticostriatal interactions. In this sum- mary of topics, which is not meant to provide a comprehensive survey, we will first review the anatomy of corticostriatal circuits, comparing different ways by which “loops” of cortical–basal ganglia circuits communicate. Next, we will address the causal importance and systems-neurophysiological mechanisms of corticostriatal interactions for memory, emphasizing the communi- cation between hippocampus and ventral striatum during contextual conditioning. Furthermore, ensemble recording techniques have been applied to compare information processing in the dorsal and ventral striatum to predictions from reinforcement learning theory. -

Cortical Layers: What Are They Good For? Neocortex
Cortical Layers: What are they good for? Neocortex L1 L2 L3 network input L4 computations L5 L6 Brodmann Map of Cortical Areas lateral medial 44 areas, numbered in order of samples taken from monkey brain Brodmann, 1908. Primary visual cortex lamination across species Balaram & Kaas 2014 Front Neuroanat Cortical lamination: not always a six-layered structure (archicortex) e.g. Piriform, entorhinal Larriva-Sahd 2010 Front Neuroanat Other layered structures in the brain Cerebellum Retina Complexity of connectivity in a cortical column • Paired-recordings and anatomical reconstructions generate statistics of cortical connectivity Lefort et al. 2009 Information flow in neocortical microcircuits Simplified version “computational Layer 2/3 layer” Layer 4 main output Layer 5 main input Layer 6 Thalamus e - excitatory, i - inhibitory Grillner et al TINS 2005 The canonical cortical circuit MAYBE …. DaCosta & Martin, 2010 Excitatory cell types across layers (rat S1 cortex) Canonical models do not capture the diversity of excitatory cell classes Oberlaender et al., Cereb Cortex. Oct 2012; 22(10): 2375–2391. Coding strategies of different cortical layers Sakata & Harris, Neuron 2010 Canonical models do not capture the diversity of firing rates and selectivities Why is the cortex layered? Do different layers have distinct functions? Is this the right question? Alternative view: • When thinking about layers, we should really be thinking about cell classes • A cells class may be defined by its input connectome and output projectome (and some other properties) • The job of different classes is to (i) make associations between different types of information available in each cortical column and/or (ii) route/gate different streams of information • Layers are convenient way of organising inputs and outputs of distinct cell classes Excitatory cell types across layers (rat S1 cortex) INTRATELENCEPHALIC (IT) | PYRAMIDAL TRACT (PT) | CORTICOTHALAMIC (CT) From Cereb Cortex. -

Perspectives
PERSPECTIVES reptiles, to birds and mammals, to primates OPINION and, finally, to humans — ascending from ‘lower’ to ‘higher’ intelligence in a chrono- logical series. They believed that the brains Avian brains and a new understanding of extant vertebrates retained ancestral structures, and, therefore, that the origin of of vertebrate brain evolution specific human brain subdivisions could be traced back in time by examining the brains of extant non-human vertebrates. In The Avian Brain Nomenclature Consortium* making such comparisons, they noted that the main divisions of the human CNS — Abstract | We believe that names have a pallium is nuclear, and the mammalian the spinal cord, hindbrain, midbrain, thala- powerful influence on the experiments we cortex is laminar in organization, the avian mus, cerebellum and cerebrum or telen- do and the way in which we think. For this pallium supports cognitive abilities similar cephalon — were present in all vertebrates reason, and in the light of new evidence to, and for some species more advanced than, (FIG. 1a). Edinger, however, noted that the about the function and evolution of the those of many mammals. To eliminate these internal organization of the telencephala vertebrate brain, an international consortium misconceptions, an international forum of showed the most pronounced differences of neuroscientists has reconsidered the neuroscientists (BOX 1) has, for the first time between species. In mammals, the outer traditional, 100-year-old terminology that is in 100 years, developed new terminology that part of the telencephalon was found to have used to describe the avian cerebrum. Our more accurately reflects our current under- prominently layered grey matter (FIG. -

Normal Mentality Associated with a Maldeveloped " Rhinencephalon " by P
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.13.3.191 on 1 August 1950. Downloaded from J. Neurol. Neurosurg. Psychiat., 1950, 13, 191. NORMAL MENTALITY ASSOCIATED WITH A MALDEVELOPED " RHINENCEPHALON " BY P. W. NATHAN and MARION C. SMITH Fronm the Neurological Research Unit of the Medical Research Coulncil, National Hospital. Queen Square, London In 1937 Papez published his views on the Case Report functions of the gyrus fornicatus. He summarized The specimen is the brain of a man, aged 34, who died them as follows. of a chondrosarcoma of the ileum. He was one of a series of cases under investigation in a programme of " It is proposed that the hypothalamus, the anterior thalamic nuclei, the gyrus cinguli, the hippocampus, research concerned with operations for the relief of and their inter-connexions constitute a harmonious pain. As many hours had been devoted to the testing mechanism which may elaborate the functions of of sensation and the discussion of signs and symptoms, central emotion, as well as participate in emotional our knowledge of the patient, which extended over a expression." period of six months, was much greater than that Bilateral temporal lobectomies performed on acquired in most routine cases. Protected by copyright. and Bucy in 1939 gave evidence No abnormality of development had been suspected monkeys by Kiuver during the patient's life, for he came well within normal supporting Papez' general hypothesis. The effects limits from intellectual and psychological points of of extensive lesions of the hippocampus-fornix view. His mother had remained well during pregnancy, system in cats and dogs were reported by Spiegel, and his birth was normal. -

Poumrnai of Anatomp Anb Piloto
poumrnaI of anatomp anb pilotO. NOTES UPON THE NATURAL SUBDIVISION OF THE CEREBRAL HEMISPHERE. By G. ELLIOT SMITH, M.D., Fellow of St John's College, Cambridge; Professor ofAnatomy, Cairo. IT is a peculiar fact, significant not only of the imperfections of the current nomenclature, but even to a greater extent of the unsatisfactory state of the present teaching in cerebral morphology, that there is no term generally accepted or acceptable among the multitude of names now employed in Descriptive Anatomy which can be applied exclusively and without confusion to the most characteristic and distinctive feature of the mammalian brain; to that part, in fact, which is the dominant organ of the whole body, and in the more highly placed Eutheria, constitutes the great bulk of the whole nervous system. I refer to that area of the cerebral cortex, with its associated medullary matter, which, in a series of earlier memoirs,' I have wrongly called the " pallium." But it is only one of the three histological formations which constitute the true pallium; and, as it is the latest of these to reach the height of its development, we. may call it the " new pallium," or, if the hybrid term be permissible, " neopallium," in contradistinction to the " old pallium " of the Sauropsida and the earlier Verte- brata, which is chiefly formed of the other two pallial areas. If a cerebral hemisphere of any mammal be submitted to careful examination, it will be found to be composed of a number of distinct regions, each of which exhibits well-defined and unmistakable histological features peculiar to itself. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

The Importance of Hippocampal Volume Reduction in People with Alzheimer’S Disease
Journal of ISSN: 2581-7388 Biomedical Research and Reviews Volume 1: 2 J Biomed Res Rev 2018 The importance of Hippocampal Volume Reduction in People with Alzheimer’s Disease 1Department of Neurosurgery, Federal University of São Paulo, Brazil 1* Mirto Nelso Prandini 2Department of Medicine, University of Medicine of ABC- FMABC, Brazil Mahara Barbosa Nonato2 3Department of Medicine(Medical Student), University of Grandes Lagos- Balestrieri João Vitor Lois3 UNILAGO, Brazil Abstract Article Information Hippocampus is an anatomic structure located inside the temporal Article Type: Research lobes. It is and an important component of the limbic system and considered the main place of memory. It plays, also, an important role Article Number: JBRR110 Received Date: 26 April, 2018 in the visuospatial memory. The healthy aging process is accompanied Accepted Date: 30 May, 2018 by the decline of physiological functions as well as some cognitive Published Date: 06 June, 2018 abilities mainly the ones related with episodic memory, the principal function carried out by the hippocampus. In senesce or in cases of *Corresponding author: Dr. Mirto Nelso Prandini, pathological process, some anatomic alterations in neural structures Department of Neurosurgery, Federal University of São may be encountered such as the reduction of the cerebral volume as a Paulo, Rua dos Crisântemos, 117 CEP 04049 020, São Paulo whole. Therefore, one of the principal challenges in clinical practice is Brazil. Tel: + 991156830; Email: mnprandini(at)uol.com.br to separate the normal to the pathological cognition when occurring in old aged people as well as in patients suffering from diseases located Citation: Prandini MN, Nonata MB, Lois BJV (2018) The in the hippocampus.