4 Bayer Exp Neurol 107 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Non-Canonical Feedforward Pathway for Computing Odor Identity
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.317248; this version posted September 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A non-canonical feedforward pathway for computing odor identity Honggoo Chae1♯, Arkarup Banerjee1,2,3♯ & Dinu F. Albeanu1,2* 1 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 2 Cold Spring Harbor Laboratory School for Biological Sciences, Cold Spring Harbor, NY 3 current address - New York University Medical Center, New York, NY ♯ equal contribution * Correspondence: [email protected] Short title: Cell-type specific decoding of odor identity and intensity in the olfactory bulb Key words: mitral and tufted cells, piriform cortex, anterior olfactory nucleus, cortical feedback, concentration invariant odor identity decoding, two photon calcium imaging, PCA, dPCA, linear and non-linear decoders bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.317248; this version posted September 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Sensory systems rely on statistical regularities in the experienced inputs to either group disparate stimuli, or parse them into separate categories1,2. While considerable progress has been made in understanding invariant object recognition in the visual system3–5, how this is implemented by olfactory neural circuits remains an open question6–10. The current leading model states that odor identity is primarily computed in the piriform cortex, drawing from mitral cell (MC) input6–9,11. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Limbic Areas Have Been Quantitatively Stud Tex: an Outer Layer of Small Granular Type Cells, An
CHAPTER 15 Development of the Limbic Cortical Areas 15.1 Neurogenetic Gradients in the Lateral Limbic 15.2.3 The Retrosplenial Area, 195 Areas, 187 15.2.4 Gradients Between Areas, 197 15.1.1 Radial and Transverse Neurogenetic 15.3 Neurogenetic Gradients in- the Orbital Cortex, Gradients, 188 197 15.1.2 Unique Longitudinal Neurogenetic 15.4 Correlations Between Neurogenetic Gradients Gradients, 188 and Anatomical Connections in the Limbic 15.2 Neurogenetic Gradients in the Medial Limbic Cortex, 198 Areas, 191 15.4.1 Connections of the Anterior Thalamic 15.2.1 The Dorsal Peduncular and Infralimbic Nuclear Complex with the Medial Limbic Areas, 191 Cortex, 198 15.2.2 The Cingulate Areas, 193 15.4.2 Thalamocortical Connections of the Gustatory Cortex, 198 As early as 1664, Thomas Willis wrote that the cortex hypothesis and named the interconnected cortical and on the borders of the cerebral hemispheres had unique subcortical structures the limbic system. Since then, anatomical features resembling a "hem" or "limbus." anatomical and functional links in the limbic system in In his 1878 paper, Broca called that area the "grande general and the medial limbic cortex in particular have lobe limbique" (reviewed in White, 1965). In rats as been widely studied in relation to behavior (Isaacson, well as in man, the limbic lobe is a continuous cortical 1974) and support has been provided for Papez' orig band encircling the neocortex. In this chapter, we use inal hypothesis. The participation of the lateral limbic the term limbic cortex to include parts of Broca's cortex in other circuits involved with emotional be limbic lobe that have been traditionally considered havior was' emphasized by Livingston and Escobar neocortex by some (Papez, 1937; MacLean, 1952; (1971). -

The Evolutionary Development of the Brain As It Pertains to Neurosurgery
Open Access Original Article DOI: 10.7759/cureus.6748 The Evolutionary Development of the Brain As It Pertains to Neurosurgery Jaafar Basma 1 , Natalie Guley 2 , L. Madison Michael II 3 , Kenan Arnautovic 3 , Frederick Boop 3 , Jeff Sorenson 3 1. Neurological Surgery, University of Tennessee Health Science Center, Memphis, USA 2. Neurological Surgery, University of Arkansas for Medical Sciences, Little Rock, USA 3. Neurological Surgery, Semmes-Murphey Clinic, Memphis, USA Corresponding author: Jaafar Basma, [email protected] Abstract Background Neuroanatomists have long been fascinated by the complex topographic organization of the cerebrum. We examined historical and modern phylogenetic theories pertaining to microneurosurgical anatomy and intrinsic brain tumor development. Methods Literature and history related to the study of anatomy, evolution, and tumor predilection of the limbic and paralimbic regions were reviewed. We used vertebrate histological cross-sections, photographs from Albert Rhoton Jr.’s dissections, and original drawings to demonstrate the utility of evolutionary temporal causality in understanding anatomy. Results Phylogenetic neuroanatomy progressed from the substantial works of Alcmaeon, Herophilus, Galen, Vesalius, von Baer, Darwin, Felsenstein, Klingler, MacLean, and many others. We identified two major modern evolutionary theories: “triune brain” and topological phylogenetics. While the concept of “triune brain” is speculative and highly debated, it remains the most popular in the current neurosurgical literature. Phylogenetics inspired by mathematical topology utilizes computational, statistical, and embryological data to analyze the temporal transformations leading to three-dimensional topographic anatomy. These transformations have shaped well-defined surgical planes, which can be exploited by the neurosurgeon to access deep cerebral targets. The microsurgical anatomy of the cerebrum and the limbic system is redescribed by incorporating the dimension of temporal causality. -

Cortical and Subcortical Anatomy: Basics and Applied
43rd Congress of the Canadian Neurological Sciences Federation Basic mechanisms of epileptogenesis and principles of electroencephalography Cortical and subcortical anatomy: basics and applied J. A. Kiernan MB, ChB, PhD, DSc Department of Anatomy & Cell Biology, The University of Western Ontario London, Canada LEARNING OBJECTIVES Know and understand: ! Two types of principal cell and five types of interneuron in the cerebral cortex. ! The layers of the cerebral cortex as seen in sections stained to show either nucleic acids or myelin. ! The types of corrtex: allocortex and isocortex. ! Major differences between extreme types of isocortex. As seen in primary motor and primary sensory areas. ! Principal cells in different layers give rise to association, commissural, projection and corticothalamic fibres. ! Cortical neurons are arranged in columns of neurons that share the same function. ! Intracortical circuitry provides for neurons in one column to excite one another and to inhibit neurons in adjacent columns. ! The general plan of neuronal connections within nuclei of the thalamus. ! The location of motor areas of the cerebral cortex and their parallel and hierarchical projections to the brain stem and spinal cord. ! The primary visual area and its connected association areas, which have different functions. ! Somatotopic representation in the primary somatosensory and motor areas. ! Cortical areas concerned with perception and expression of language, and the anatomy of their interconnections. DISCLOSURE FORM This disclosure form must be included as the third page of your Course Notes and the third slide of your presentation. It is the policy of the Canadian Neurological Sciences Federation to insure balance, independence, objectivity and scientific rigor in all of its education programs. -

Neuronal Properties and Synaptic Connectivity in Rodent Presubiculum Jean Simonnet
Neuronal properties and synaptic connectivity in rodent presubiculum Jean Simonnet To cite this version: Jean Simonnet. Neuronal properties and synaptic connectivity in rodent presubiculum. Neurons and Cognition [q-bio.NC]. Université Pierre et Marie Curie - Paris VI, 2014. English. NNT : 2014PA066435. tel-02295014 HAL Id: tel-02295014 https://tel.archives-ouvertes.fr/tel-02295014 Submitted on 24 Sep 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE DE DOCTORAT DE L’UNIVERSITÉ PIERRE ET MARIE CURIE Spécialité Neurosciences École doctorale Cerveau – Cognition – Comportement Présentée par : Jean Simonnet Pour obtenir le grade de DOCTEUR DE L’UNIVERSITÉ PIERRE ET MARIE CURIE Sujet de la thèse : Neuronal properties and synaptic connectivity in rodent presubiculum Soutenue le 23.09.2014 devant le jury composé de : Dr Jean-Christophe Poncer Président Dr Dominique Debanne Rapporteur Dr Maria Cecilia Angulo Rapportrice Pr Hannah Monyer Examinatrice Dr Bruno Cauli Examinateur Dr Desdemona Fricker Directrice de thèse Université Pierre & Marie Curie - Paris 6 Tél. Secrétariat : 01 42 34 68 35 Bureau d’accueil, inscription des doctorants Fax : 01 42 34 68 40 et base de données Tél. pour les étudiants de A à EL : 01 42 34 68 41 Esc. -

Sequential Pattern of Sublayer Formation in the Paleocortex and Neocortex
Medical Molecular Morphology (2020) 53:168–176 https://doi.org/10.1007/s00795-020-00245-7 ORIGINAL PAPER Sequential pattern of sublayer formation in the paleocortex and neocortex Makoto Nasu1 · Kenji Shimamura2 · Shigeyuki Esumi1 · Nobuaki Tamamaki1 Received: 17 December 2019 / Accepted: 13 January 2020 / Published online: 30 January 2020 © The Japanese Society for Clinical Molecular Morphology 2020 Abstract The piriform cortex (paleocortex) is the olfactory cortex or the primary cortex for the sense of smell. It receives the olfac- tory input from the mitral and tufted cells of the olfactory bulb and is involved in the processing of information pertaining to odors. The piriform cortex and the adjoining neocortex have diferent cytoarchitectures; while the former has a three-layered structure, the latter has a six-layered structure. The regulatory mechanisms underlying the building of the six-layered neo- cortex are well established; in contrast, less is known about of the regulatory mechanisms responsible for structure formation of the piriform cortex. The diferences as well as similarities in the regulatory mechanisms between the neocortex and the piriform cortex remain unclear. Here, the expression of neocortical layer-specifc genes in the piriform cortex was examined. Two sublayers were found to be distinguished in layer II of the piriform cortex using Ctip2/Bcl11b and Brn1/Pou3f3. The sequential expression pattern of Ctip2 and Brn1 in the piriform cortex was similar to that detected in the neocortex, although the laminar arrangement in the piriform cortex exhibited an outside-in arrangement, unlike that observed in the neocortex. Keywords Piriform cortex (paleocortex) · Sublayer · Sequential expression · Ctip2/Bcl11b · Brn1/Pou3f3 Introduction six-layered cortex, which is a mammal-specifc feature, are well established; sequential expression of transcription fac- The piriform cortex (paleocortex) is the olfactory cortex or tors is involved in determining cell identities, and late-born the primary cortex for the sense of smell. -

Activation Pattern in Lower Level in the Neo-,Paleo-,And Archicortices
ACTIVATION PATTERN IN LOWER LEVEL IN THE NEO-,PALEO-,AND ARCHICORTICES Goro IMAMURA* AND Hiroshi KAWAMURA** Laboratory of Neurophysiology,Institute of Brain-Research, School of Medicine,University of Tokyo In the classical works of brain physiology,solely the neocortical electrical activity was recorded in the most cases,and two different patterns;low volt- age fast waves and slow waves with spindle bursts were classified as an indi- cator of the functional level of the brain.These two patterns,namely arousal pattern and sleep pattern of the neocortex respectively,were utilized to study the effects of various kinds of physiological procedures and the effects of drugs upon the brain. After the administration of some kinds of central depressants,especially narcotics(dial-urethane,pentobarbital etc.)often occurred a peculiar anesthe- tized pattern in the neocortex,and high voltage"arousal"pattern appeared by stimulation.For example,in the works of MURPHY & GELLHORN(1945)1), electrical stimulation of the posterior hypothalamus did not produce ordinary low voltage fast waves in the neocortex under dial-urethane anesthesia,and instead,4-8 cps high amplitude sinusoidal waves were elicited.SLOAN et al. (1950)2)also showed that electrical stimulation of the anterior limbic lobe elicited in some cases high voltage"arousal"pattern in the neocortical area. Similar pattern was also observed in the paleo-cortex(pyriform lobe)and its subcortical nuclei(amygdala),by MACLEAN et al.(1952)3)and described as olfactory-like responses.FEINDEL & GLOOR(1954)"also described 12-16 cps high amplitude waves as"arousal"pattern in the amygdala under pento- barbital anesthesia. In the present paper,the observation of this peculiar pattern in the neo-, paleo-,and archicortices was made and named as the activation pattern in lower level,and the conditions for appearance of this pattern were discussed. -

Multimodal Mapping and Analysis of the Cyto- and Receptorarchitecture of the Human Hippocampus
Brain Structure and Function (2020) 225:881–907 https://doi.org/10.1007/s00429-019-02022-4 ORIGINAL ARTICLE Multimodal mapping and analysis of the cyto‑ and receptorarchitecture of the human hippocampus Nicola Palomero‑Gallagher1,2,3 · Olga Kedo1 · Hartmut Mohlberg1 · Karl Zilles1,4 · Katrin Amunts1,3 Received: 19 June 2019 / Accepted: 26 December 2019 / Published online: 18 January 2020 © The Author(s) 2020 Abstract The human hippocampal formation is relevant for various aspects of memory and learning, and the diferent hippocampal regions are diferentially afected by neuropsychiatric disorders. Therefore, the hippocampal formation has been subject of numerous cytoarchitectonic and other mapping studies, which resulted in divergent parcellation schemes. To understand the principles of hippocampal architecture, it is necessary to integrate diferent levels of hippocampal organisation, going beyond one modality. We here applied a multimodal mapping approach combining cyto- and multi-receptorarchitectonic analyses, and generated probabilistic maps in stereotaxic space of the identifed regions. Cytoarchitecture in combination with the regional and laminar distribution of 15 neurotransmitter receptors visualized by in vitro receptor autoradiography were analysed in seven hemispheres from 6 unfxed shock frozen and serially sectioned brains. Cytoarchitectonic delineations for generation of probabilistic maps were carried out on histological sections from ten fxed, parafn embedded and serially sectioned brains. Nine cyto- and receptorarchitectonically distinct regions were identifed within the hippocampal formation (i.e., fascia dentata, cornu Ammonis (CA) regions 1–4, prosubiculum, subiculum proper, presubiculum and parasubiculum), as well as the hippocampal-amygdaloid transition area and the periallocortical transsubiculum. Subsequently generated probabilistic maps quantify intersubject variability in the size and extent of these cyto- and receptorarchitectonically distinct regions. -

History, Anatomical Nomenclature, Comparative Anatomy and Functions of the Hippocampal Formation
Bratisl Lek Listy 2006; 107 (4): 103106 103 TOPICAL REVIEW History, anatomical nomenclature, comparative anatomy and functions of the hippocampal formation El Falougy H, Benuska J Institute of Anatomy, Faculty of Medicine, Comenius University, Bratislava, [email protected] Abstract The complex structures in the cerebral hemispheres is included under one term, the limbic system. Our conception of this system and its special functions rises from the comparative neuroanatomical and neurophysiological studies. The components of the limbic system are the hippocampus, gyrus parahippocampalis, gyrus dentatus, gyrus cinguli, corpus amygdaloideum, nuclei anteriores thalami, hypothalamus and gyrus paraterminalis Because of its unique macroscopic and microscopic structure, the hippocampus is a conspicuous part of the limbic system. During phylogenetic development, the hippocampus developed from a simple cortical plate in amphibians into complex three-dimensional convoluted structure in mammals. In the last few decades, structures of the limbic system were extensively studied. Attention was directed to the physi- ological functions and pathological changes of the hippocampus. Experimental studies proved that the hippocampus has a very important role in the process of learning and memory. Another important functions of the hippocampus as a part of the limbic system is its role in regulation of sexual and emotional behaviour. The term hippocampal formation is defined as the complex of six structures: gyrus dentatus, hippocampus proprius, subiculum proprium, presubiculum, parasubiculum and area entorhinalis In this work we attempt to present a brief review of knowledge about the hippocampus from the point of view of history, anatomical nomenclature, comparative anatomy and functions (Tab. 1, Fig. 2, Ref. -
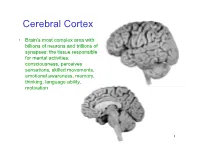
Cerebral Cortex
Cerebral Cortex • Brain’s most complex area with billions of neurons and trillions of synapses: the tissue responsible for mental activities: consciousness, perceives sensations, skilled movements, emotional awareness, memory, thinking, language ability, motivation 1 John Lorber, Science 210:1232 (1980) “There a young student at this university who has an IQ of 126, has gained a first-class honors degree in mathematics, and is socially completely normal. And yet the boy has virtually no brain.” “The student’s physician at the university noticed that the student had slightly larger than normal head… When we did a brain scan on him we saw that instead of the normal 4.5 cm thickness of brain tissue there was just a millimeter or so. His cranium is filled with CSF.” How is this possible? What does it tell us? Do you think this would be OK if it happened to an adult? To a 15 year old? To a 5 year old? To a neonate? 3 Types of Cerebral Cortex • Neocortex – Newest in evolution – About 90% of total – 6 layers, most complex • Paleocortex – Associated with olfactory system, the parahippocampal gyrus, uncus – fewer than 6 layers • Archicortex – Hippocampal formation; limbic system – 3 layers, most primitive • Mesocortex – Cingulate gyrus, insular cortex – Transitional between archicortex and neocortex 5 The perks of having a neocortex • The words used to describe the higher mental capacities of animals with a large neocortex include: – CONSCIOUSNESS – FREE WILL – INTELLIGENCE – INSIGHT • Animals with much simpler brains learn well, so LEARNING should not be among these capacities (Macphail 1982). • A species could have genetically determined mechanisms, acquired through evolutionary selection, for taking advantage of the regular features of the environment, or they could have learned through direct experience.