Pharmaceutical Properties and Applications of a Natural Polymer from Grewia Mollis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Article Pharmaceutical Properties and Applications of a Natural Polymer from Grewia Mollis
Hindawi Publishing Corporation Journal of Polymers Volume 2013, Article ID 938726, 8 pages http://dx.doi.org/10.1155/2013/938726 Review Article Pharmaceutical Properties and Applications of a Natural Polymer from Grewia mollis Elijah I. Nep, Patricia O. Odumosu, Ndidi C. Ngwuluka, Patrick O. Olorunfemi, and Nelson A. Ochekpe Biomaterials and Drug Delivery Research Group, Faculty of Pharmaceutical Sciences, University of Jos, PMB 2084, Jos 930001, Nigeria Correspondence should be addressed to Elijah I. Nep; [email protected] Received 22 March 2013; Accepted 16 June 2013 Academic Editor: Alain Durand Copyright © 2013 Elijah I. Nep et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The use of naturally occurring biocompatible materials has been the focus of recent research activity in the design of dosage forms for immediate and controlled release formulations. Grewia gum is an intracellular gum obtained by extraction from the inner stem bark of the shrub Grewia mollis (Malvaceae). It grows abundantly (wild or cultivated) in the middle belt region of Nigeria, andthemucilagehasbeenusedbyindigenesofthisbeltasthickenerinsoups.Grewiagumhasbeeninvestigatedforpotential applications in pharmaceutical dosage forms. The industrial extrapolation of the applications of the gum has, however, been slowed by the limited structural, toxicological, and stability data available on the gum. This paper highlights ethnobotanical uses of G. mollis shrub and discusses the structural features, functional properties, and applications of grewia gum with emphases on its pharmaceutical potentials. 1. Introduction 2. Grewia mollis Plant Plant materials are playing increasing role as alternatives to G. -

Checklist of Vascular Plants Recorded for Cattana Wetlands Class Family Code Taxon Common Name
Checklist of Vascular Plants Recorded for Cattana Wetlands Class Family Code Taxon Common Name FERNS & ALLIES Aspleniaceae Asplenium nidus Birds Nest Fern Blechnaceae Stenochlaena palustris Climbing Swamp Fern Dryopteridaceae Coveniella poecilophlebia Marsileaceae Marsilea mutica Smooth Nardoo Polypodiaceae Colysis ampla Platycerium hillii Northern Elkhorn Fern Pteridaceae Acrostichum speciosum Mangrove Fern Schizaeaceae Lygodium microphyllum Climbing Maidenhair Fern Lygodium reticulatum GYMNOSPERMS Araucariaceae Agathis robusta Queensland Kauri Pine Podocarpaceae Podocarpus grayae Weeping Brown Pine FLOWERING PLANTS-DICOTYLEDONS Acanthaceae * Asystasia gangetica subsp. gangetica Chinese Violet Pseuderanthemum variabile Pastel Flower * Sanchezia parvibracteata Sanchezia Amaranthaceae * Alternanthera brasiliana Brasilian Joyweed * Gomphrena celosioides Gomphrena Weed; Soft Khaki Weed Anacardiaceae Blepharocarya involucrigera Rose Butternut * Mangifera indica Mango Tuesday, 31 August 2010 Checklist of Plants for Cattana Wetlands RLJ Page 1 of 12 Class Family Code Taxon Common Name Semecarpus australiensis Tar Tree Annonaceae Cananga odorata Woolly Pine Melodorum leichhardtii Acid Drop Vine Melodorum uhrii Miliusa brahei Raspberry Jelly Tree Polyalthia nitidissima Canary Beech Uvaria concava Calabao Xylopia maccreae Orange Jacket Apocynaceae Alstonia scholaris Milky Pine Alyxia ruscifolia Chain Fruit Hoya pottsii Native Hoya Ichnocarpus frutescens Melodinus acutiflorus Yappa Yappa Tylophora benthamii Wrightia laevis subsp. millgar Millgar -

Introduction: the Tiliaceae and Genustilia
Cambridge University Press 978-0-521-84054-5 — Lime-trees and Basswoods Donald Pigott Excerpt More Information Introduction: the 1 Tiliaceae and genus Tilia Tilia is the type genus of the family name Tiliaceae Juss. (1789), The ovary is syncarpous with five or more carpels but only and T. × europaea L.thetypeofthegenericname(Jarviset al. one style and a stigma with a lobe above each carpel. In Tili- 1993). Members of Tiliaceae have many morphological char- aceae, the ovules are anatropous. In Malvaceae, filaments of acters in common with those of Malvaceae Juss. (1789) and the stamens are fused into a tube but have separate apices that both families were placed in the order Malvales by Engler each bear a unilocular anther. Staminodes are absent. Each of (1912). In Engler’s treatment, Tiliaceae consisted mainly of five or more carpels supports a separate style, which together trees and shrubs belonging to several genera, including a pass through the staminal tube so that the stigmas are exposed few herbaceous genera, almost all occurring in the warmer above the anthers. The ovules may be either anatropous or regions. campylotropous. This treatment was revised by Engler and Diels (1936). The Molecular studies comprising sequence analysis of DNA of family was retained by Cronquist (1981) and consisted of about two plastid genes (Bayer et al. 1999) show that, in general, the 50 genera and 700 species distributed in the tropics and warmer inclusion of most genera, including Tilia, traditionally placed parts of the temperate zones in Asia, Africa, southern Europe in Malvales is correct. There is, however, clear evidence that and America. -

Corchorus L. and Hibiscus L.: Molecular Phylogeny Helps to Understand Their Relative Evolution and Dispersal Routes
Corchorus L. and Hibiscus L.: Molecular Phylogeny Helps to Understand Their Relative Evolution and Dispersal Routes Arif Mohammad Tanmoy1, Md. Maksudul Alam1,2, Mahdi Muhammad Moosa1,3, Ajit Ghosh1,4, Waise Quarni1,5, Farzana Ahmed1, Nazia Rifat Zaman1, Sazia Sharmin1,6, Md. Tariqul Islam1, Md. Shahidul Islam1,7, Kawsar Hossain1, Rajib Ahmed1 and Haseena Khan1* 1Molecular Biology Lab, Department of Biochemistry and Molecular Biology, University of Dhaka, Dhaka 1000, Bangladesh. 2Department of Molecular and Cell Biology, Center for Systems Biology, University of Texas at Dallas, Richardson, TX 75080, USA. 3Graduate Studies in Biological Sciences, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA. 4Plant Molecular Biology, International Centre for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi 110067, India. 5Department of Pathology and Cell Biology, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA. 6Department of Kidney Development, Institute of Molecular Embryology and Genetics, Kumamoto University, 2-2-1 Honjo, Kumamoto 860-0811, Japan. 7Breeding Division, Bangladesh Jute Research Institute (BJRI), Dhaka 1207, Bangladesh. ABSTRACT: Members of the genera Corchorus L. and Hibiscus L. are excellent sources of natural fibers and becoming much important in recent times due to an increasing concern to make the world greener. The aim of this study has been to describe the molecular phylogenetic relationships among the important members of these two genera as well as to know their relative dispersal throughout the world. Monophyly of Corchorus L. is evident from our study, whereas paraphyletic occurrences have been identified in case of Hibiscus L. -

Alien Flora of Europe: Species Diversity, Temporal Trends, Geographical Patterns and Research Needs
Preslia 80: 101–149, 2008 101 Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs Zavlečená flóra Evropy: druhová diverzita, časové trendy, zákonitosti geografického rozšíření a oblasti budoucího výzkumu Philip W. L a m b d o n1,2#, Petr P y š e k3,4*, Corina B a s n o u5, Martin H e j d a3,4, Margari- taArianoutsou6, Franz E s s l7, Vojtěch J a r o š í k4,3, Jan P e r g l3, Marten W i n t e r8, Paulina A n a s t a s i u9, Pavlos A n d r i opoulos6, Ioannis B a z o s6, Giuseppe Brundu10, Laura C e l e s t i - G r a p o w11, Philippe C h a s s o t12, Pinelopi D e l i p e t - rou13, Melanie J o s e f s s o n14, Salit K a r k15, Stefan K l o t z8, Yannis K o k k o r i s6, Ingolf K ü h n8, Hélia M a r c h a n t e16, Irena P e r g l o v á3, Joan P i n o5, Montserrat Vilà17, Andreas Z i k o s6, David R o y1 & Philip E. H u l m e18 1Centre for Ecology and Hydrology, Hill of Brathens, Banchory, Aberdeenshire AB31 4BW, Scotland, e-mail; [email protected], [email protected]; 2Kew Herbarium, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3AB, United Kingdom; 3Institute of Bot- any, Academy of Sciences of the Czech Republic, CZ-252 43 Průhonice, Czech Republic, e-mail: [email protected], [email protected], [email protected], [email protected]; 4Department of Ecology, Faculty of Science, Charles University, Viničná 7, CZ-128 01 Praha 2, Czech Republic; e-mail: [email protected]; 5Center for Ecological Research and Forestry Applications, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain, e-mail: [email protected], [email protected]; 6University of Athens, Faculty of Biology, Department of Ecology & Systematics, 15784 Athens, Greece, e-mail: [email protected], [email protected], [email protected], [email protected], [email protected]; 7Federal Environment Agency, Department of Nature Conservation, Spittelauer Lände 5, 1090 Vienna, Austria, e-mail: [email protected]; 8Helmholtz Centre for Environmental Research – UFZ, Department of Community Ecology, Theodor-Lieser- Str. -

Downloaded from Brill.Com10/07/2021 08:53:11AM Via Free Access 130 IAWA Journal, Vol
IAWA Journal, Vol. 27 (2), 2006: 129–136 WOOD ANATOMY OF CRAIGIA (MALVALES) FROM SOUTHEASTERN YUNNAN, CHINA Steven R. Manchester1, Zhiduan Chen2 and Zhekun Zhou3 SUMMARY Wood anatomy of Craigia W.W. Sm. & W.E. Evans (Malvaceae s.l.), a tree endemic to China and Vietnam, is described in order to provide new characters for assessing its affinities relative to other malvalean genera. Craigia has very low-density wood, with abundant diffuse-in-aggre- gate axial parenchyma and tile cells of the Pterospermum type in the multiseriate rays. Although Craigia is distinct from Tilia by the pres- ence of tile cells, they share the feature of helically thickened vessels – supportive of the sister group status suggested for these two genera by other morphological characters and preliminary molecular data. Although Craigia is well represented in the fossil record based on fruits, we were unable to locate fossil woods corresponding in anatomy to that of the extant genus. Key words: Craigia, Tilia, Malvaceae, wood anatomy, tile cells. INTRODUCTION The genus Craigia is endemic to eastern Asia today, with two species in southern China, one of which also extends into northern Vietnam and southeastern Tibet. The genus was initially placed in Sterculiaceae (Smith & Evans 1921; Hsue 1975), then Tiliaceae (Ren 1989; Ying et al. 1993), and more recently in the broadly circumscribed Malvaceae s.l. (including Sterculiaceae, Tiliaceae, and Bombacaceae) (Judd & Manchester 1997; Alverson et al. 1999; Kubitzki & Bayer 2003). Similarities in pollen morphology and staminodes (Judd & Manchester 1997), and chloroplast gene sequence data (Alverson et al. 1999) have suggested a sister relationship to Tilia. -

Microcos Antidesmifolia (Malvaceae-Grewioideae), a Poorly Known Species in Singapore
Gardens' Bulletin Singapore 72(2): 159–164. 2020 159 doi: 10.26492/gbs72(2).2020-04 Microcos antidesmifolia (Malvaceae-Grewioideae), a poorly known species in Singapore S.K. Ganesan1, R.C.J. Lim2, P.K.F. Leong1 & X.Y. Ng2 1Singapore Botanic Gardens, National Parks Board, 1 Cluny Road, 259569 Singapore [email protected] 2 Native Plant Centre, Horticulture and Community Gardening Division, National Parks Board, 100K Pasir Panjang Road, 118526 Singapore ABSTRACT. A poorly known species in Singapore, Microcos antidesmifolia (King) Burret, is described and illustrated for the first time. In Singapore, it is known from the type variety, Microcos antidesmifolia (King) Burret var. antidesmifolia. Notes on distribution, ecology and conservation status are given. This species is assessed as Critically Endangered for Singapore. A key is given for the fiveMicrocos L. species in Singapore. Keywords. Conservation assessment, distribution, ecology, flora Introduction The genus Microcos L. comprises about 80 species that are distributed in tropical Africa (not in Madagascar), India, Sri Lanka, Myanmar, Indochina, south China and throughout Malesia (except the Lesser Sunda Islands) (Chung & Soepadmo, 2011). Until about 2007, Microcos was placed in the family Tiliaceae. However, phylogenetic analysis using both molecular and morphological data has led to the recognition of an expanded Malvaceae, composed of the formerly recognised families Malvaceae s.s., Tiliaceae, Bombacaceae and Sterculiaceae, and for the Malvaceae s.l. to be divided into nine sub-families (Alverson et al., 1999; Bayer et al., 1999; Bayer & Kubitzki, 2003). This classification was adopted by the Angiosperm Phylogeny Group (APG, 2009, 2016). Here we follow APG and consider Microcos in Malvaceae, subfamily Grewioideae Dippel. -

The Fossil Record of Angiosperm Families in Relation to Baraminology
The Proceedings of the International Conference on Creationism Volume 7 Article 31 2013 The Fossil Record of Angiosperm Families in Relation to Baraminology Roger W. Sanders Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Sanders, Roger W. (2013) "The Fossil Record of Angiosperm Families in Relation to Baraminology," The Proceedings of the International Conference on Creationism: Vol. 7 , Article 31. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol7/iss1/31 Proceedings of the Seventh International Conference on Creationism. Pittsburgh, PA: Creation Science Fellowship THE FOSSIL RECORD OF ANGIOSPERM FAMILIES IN RELATION TO BARAMINOLOGY Roger W. Sanders, Ph.D., Bryan College #7802, 721 Bryan Drive, Dayton, TN 37321 USA KEYWORDS: Angiosperms, flowering plants, fossils, baramins, Flood, post-Flood continuity criterion, continuous fossil record ABSTRACT To help estimate the number and boundaries of created kinds (i.e., baramins) of flowering plants, the fossil record has been analyzed. To designate the status of baramin, a criterion is applied that tests whether some but not all of a group’s hierarchically immediate subgroups have a fossil record back to the Flood (accepted here as near the Cretaceous-Paleogene boundary). -

LEAF ARCHITECTURE of SELECTED SPECIES of MALVACEAE Sensu APG and ITS TAXONOMIC SIGNIFICANCE
Philippine Journal of Systematic Biology Vol. IV (June 2010) LEAF ARCHITECTURE OF SELECTED SPECIES OF MALVACEAE sensu APG AND ITS TAXONOMIC SIGNIFICANCE ALLEN ANTHONY P. LARAÑO, AND INOCENCIO E. BUOT JR. Institute of Biological Sciences, University of the Philippines Los Baños ABSTRACT The leaf architecture of Malvaceae sensu APG was examined and characterized to determine if it can be used in classification of the family and the identification of its species. Forty species were observed, measured and described. A dichotomous key was constructed based solely on leaf architecture characters. The dichotomous key indicated that leaf architecture characters can be used in distinguishing some species of Malvaceae sensu APG. Some basic leaf architectural characters can also be used in describing certain clades within the family. It is recommended that specimens are collected personally instead on relying on available specimens in the herbarium. Preparation of leaf skeletons through clearing method can also be done in future studies. Increase of sample size is also recommended. KEYWORDS: leaf architecture, APG, classification INTRODUCTION Malvaceae Jussieu, nom. cons is a newly circumscribed family of the Angiosperm Phylogeny Group (APG, 2003). This family now comprises 243 genera and 4225 species which are mainly tropical in distribution. In the APG system, member families of Malvales like Sterculiaceae, Bombacaceae, Tiliaceae and Malvaceae sensu strictu were merged to become Malvaceae sensu APG (or lato). This lumping of families became controversial and gained criticism from some taxonomists. Cheek (2006, see also Cheek in Heywood et. al., 2007, Stevens, 2010) opts for a full dismemberment of the super family into ten separate families (Bombacaceae, Malvaceae, Sterculiaceae, Tiliaceae, Durionaceae, Brownlowiaceae Byttneriaceae, Helicteraceae, Pentapetaceae, and Sparrmanniaceae). -

Bentham and Hooker Classification Faculty Name - Dr Piyush Kumar Rai Email – [email protected]
Course - B.Sc. Botany Semester - II Paper Code - BOT GE202 Paper Name – Plant Ecology and Taxonomy Topic - Bentham and Hooker Classification Faculty Name - Dr Piyush Kumar Rai Email – [email protected] Bentham and Hooker Classification George Bentham (1800 – 1884) Joseph Hooker (1817 – 1911) It was Proposed by George Bentham ( 1800 – 1884 ) and Joseph Dalton Hooker (1817 – 1911 ) in their Genera Plantarum published during July (1862 ) & April ( 1883 ) George Bentham (1800 – 1884) and Joseph Dalton Hooker ( 1817 ) – 1911) , the two British botanist who were associated with the Royal Botanic garden at kew ( England) gave most important and easily workable system of classification of angiosperms and published it in three volume of ‘Genera plantarum ‘ The first part of Genera plantarum appeared in July 1882 and the last part in April 1883 . This was the greatest taxonomic work ever produced in the united kingdom and ever since been an inspiration to generations of kew botanists . Although Bentham and Hooker’s system of classification was based on that of A.P. de Candolle but greater stress was given on contrast between free and fused petals . Their symptom was widely accepted in Britain and commonwealth countries but in Europe and North America it did not hold the much ground . Bentham and Hooker divided the seed plants into Dicotyledons, Gymnosperms and Monocotyledons. They placed Ranales in beginning and grasses at the end . The following is the summary of Bentham & Hooker’s system. DICOTYLEDONS : A . Polypetalae ( petals are free ) Series l Thalamiflorae Order 1. Ranales eg. Ranunculaceae, Magnollaceae e.t.c Order 2. Parietales eg. Papaveraceae , Cruciferae e.t.c Order 3. -
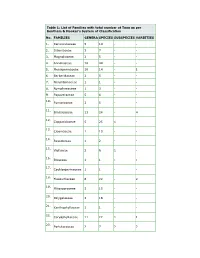
Table 1: List of Families with Total Number of Taxa As Per Bentham & Hooker’S System of Classification
Table 1: List of Families with total number of Taxa as per Bentham & Hooker’s System of Classification No. FAMILIES GENERA SPECIES SUBSPECIES VARIETIES 1. Ranunculaceae 5 14 - - 2. Dilleniaceae 3 7 - - 3. Magnoliaceae 2 5 - - 4. Annonaceae 16 44 - - 5. Menispermaceae 10 14 - 1 6. Berberidaceae 2 5 - - 7. Nelumbonaceae 1 1 - - 8. Nymphaeaceae 1 3 - - 9. Papaveraceae 5 6 - - 10. Fumariaceae 3 5 - - 11. Brassicaceae 13 24 - 4 12. Capparidaceae 5 25 1 - 13. Cleomaceae 1 10 - - 14. Resedaceae 1 2 - - 15. Violaceae 2 6 1 - 16. Bixaceae 1 1 - - 17. Cochlospermaceae 1 1 - - 18. Flacourtiaceae 8 22 - 2 19. Pittosporaceae 3 15 - - 20. Polygalaceae 3 18 - - 21. Xanthophyllaceae 1 1 - - 22. Caryophyllaceae 11 22 1 1 23. Portulacaceae 2 7 2 2 24. Tamaricaceae 1 3 - - 25. Elatinaceae 2 5 - - 26. Hypericaceae 1 7 - - 27. Clusiaceae 4 17 2 2 28. Bonnetiaceae 1 2 - - 29. Theaceae 5 6 - - 30. Actinidiaceae 1 2 - - 31. Stachyuraceae 1 1 - - 32. Dipterocarpaceae 4 9 - - 33. Ancistrocladaceae 1 1 - - 34. Malvaceae 18 64 4 - 35. Bombacaceae 5 6 - - 36. Sterculiaceae 19 43 - - 37. Tiliaceae 5 37 - - 38. Elaeocarpaceae 2 9 - - 39. Linaceae 3 4 - - 40. Erythroxylaceae 1 5 - - 41. Malpighiaceae 5 10 - - 42. Zygophyllaceae 3 5 - - 43. Geraniaceae 3 9 - - 44. Tropaeolaceae 1 2 - - 45. Limnanthaceae 1 1 - - 46. Oxalidaceae 2 17 - 6 47. Averrhoaceae 1 2 - - 48. Balsaminaceae 3 63 - 2 49. Rutaceae 23 53 - 2 50. Simaroubaceae 2 3 - - 51. Surianaceae 1 1 - - 52. Balanitaceae 1 1 - - 53. Ochnaceae 2 4 - 2 54. Burseraceae 5 9 - 1 55. Meliaceae 18 31 - 8 56. -

Thèse GOSSAN ADJA URCA 2013 100713
UFR DE PHARMACIE UFR SSMT TH SE EN COTUTELLE Année 2013 Ecole Doctorale : Sciences Technologie Santé Pour obtenir le grade de Docteur de l’Université de Reims Champagne-Ardenne (FRANCE) et de l’Université Félix Houphouët-Boigny d’Abidjan (CÔTE D’IVOIRE) Discipline : Chimie Organique des Substances Naturelles Spécialité : Pharmacognosie Par Apie Diane Patricia GOSSAN le 29 Mai 2013 Étude phytochimique de plantes médicinales issues de la flore de la Côte d’Ivoire : Gouania longipetala , Ventilago africana (Rhamnaceae), Combretum racemosum (Combretaceae) et Glyphaea brevis (Malvaceae) Directrice de thèse Pr. Laurence VOUTQUENNE-NAZABADIOKO Jury Pr. Elisabeth SEGUIN…………………………………………..Président Dr Thierry HENNEBELLE……………………………………..Rapporteur Pr. Loré Beugré Hervé César ZABRI…………………………...Rapporteur Dr Raphaël GROUGNET……………………………………….Examinateur Pr. Antoine AHIBO-COFFY……………………………………Co-directeur de thèse Dr Abdulmagid ALABDUL MAGID…………………………..Co-encadrant Dr Philomène Akoua KOUASSI-YAO……………………........Co-encadrant Pr. Laurence VOUTQUENNE-NAZABADIOKO……………..Directrice de thèse i Diane GOSSAN -Thèse de l’Université de Reims Champagne -Ardenne -2013 ii Diane GOSSAN -Thèse de l’Université de Reims Champagne -Ardenne -2013 Remerciements J’adresse mes sincères remerciements et toute ma reconnaissance à : Monsieur le Docteur Thierry HENNEBELLE Maître de Conférences à l’Université de Lille Nord de France (Lille 2) Monsieur le Professeur Loré Beugré Hervé César ZABRI, Maître de Conférences à l’Université Nangui-Abrogoua d'Abidjan Madame le Professeur Elisabeth