Coexpression of Human Somatostatin Receptor-2 (SSTR2) and SSTR3 Modulates Antiproliferative Signaling and Apoptosis Sajad a War and Ujendra Kumar*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
PDF (Appendices)
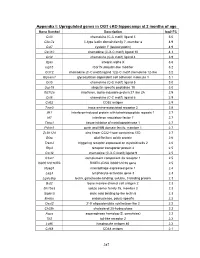
Appendix I: Upregulated genes in OGT cKO hippocampi at 2 months of age Gene Symbol Description log2 FC Ccl3 chemokine (C-C motif) ligand 3 5.0 Clec7a C-type lectin domain family 7, member a 4.9 Cst7 cystatin F (leukocystatin) 4.9 Cxcl10 chemokine (C-X-C motif) ligand 10 4.3 Ccl4 chemokine (C-C motif) ligand 4 3.9 Itgax integrin alpha X 3.6 Isg15 ISG15 ubiquitin-like modifier 3.2 Ccl12 chemokine (C-C motif) ligand 12|c-C motif chemokine 12-like 3.2 Glycam1 glycosylation dependent cell adhesion molecule 1 3.1 Ccl5 chemokine (C-C motif) ligand 5 3.0 Usp18 ubiquitin specific peptidase 18 3.0 Ifi27l2a interferon, alpha-inducible protein 27 like 2A 2.9 Ccl6 chemokine (C-C motif) ligand 6 2.9 Cd52 CD52 antigen 2.9 Taar3 trace amine-associated receptor 3 2.8 Ifit1 interferon-induced protein with tetratricopeptide repeats 1 2.7 Irf7 interferon regulatory factor 7 2.7 Timp1 tissue inhibitor of metalloproteinase 1 2.7 Pyhin1 pyrin and HIN domain family, member 1 2.7 Zc3h12d zinc finger CCCH type containing 12D 2.7 Gfap glial fibrillary acidic protein 2.6 Trem2 triggering receptor expressed on myeloid cells 2 2.6 Rtp4 receptor transporter protein 4 2.5 Cxcl9 chemokine (C-X-C motif) ligand 9 2.5 C3ar1 complement component 3a receptor 1 2.5 I830012O16Rik RIKEN cDNA I830012O16 gene 2.5 Mpeg1 macrophage expressed gene 1 2.4 Lag3 lymphocyte-activation gene 3 2.3 Lgals3bp lectin, galactoside-binding, soluble, 3 binding protein 2.3 Bst2 bone marrow stromal cell antigen 2 2.3 Slc15a3 solute carrier family 15, member 3 2.3 Siglec5 sialic acid binding Ig-like lectin 5 2.3 Endou endonuclease, polyU-specific 2.2 Oasl2 2'-5' oligoadenylate synthetase-like 2 2.2 Ch25h cholesterol 25-hydroxylase 2.2 Aspg asparaginase homolog (S. -

Arborization of Dendrites by Developing Neocortical Neurons Is Dependent on Primary Cilia and Type 3 Adenylyl Cyclase
2626 • The Journal of Neuroscience, February 6, 2013 • 33(6):2626–2638 Development/Plasticity/Repair Arborization of Dendrites by Developing Neocortical Neurons Is Dependent on Primary Cilia and Type 3 Adenylyl Cyclase Sarah M. Guadiana,1 Susan Semple-Rowland,1 Daniel Daroszewski,1 Irina Madorsky,1 Joshua J. Breunig,2 Kirk Mykytyn,3 and Matthew R. Sarkisian1 1Department of Neuroscience, McKnight Brain Institute, University of Florida, Gainesville, Florida 32610-0244, 2Cedars-Sinai Regenerative Medicine Institute, Los Angeles, California 90036, and 3Department of Pharmacology, Wexner Medical Center, Ohio State University, Columbus, Ohio 43210 The formation of primary cilia is a highly choreographed process that can be disrupted in developing neurons by overexpressing neuromodulatory G-protein-coupled receptors GPCRs or by blocking intraflagellar transport. Here, we examined the effects of overex- pressing the ciliary GPCRs, 5HT6 and SSTR3, on cilia structure and the differentiation of neocortical neurons. Neuronal overexpression of 5HT6 and SSTR3 was achieved by electroporating mouse embryo cortex in utero with vectors encoding these receptors. We found that overexpression of ciliary GPCRs in cortical neurons, especially 5HT6, induced the formation of long (Ͼ30 m) and often forked cilia. These changes were associated with increased levels of intraflagellar transport proteins and accelerated ciliogenesis in neonatal neocor- tex, the induction of which required Kif3a, an anterograde motor critical for cilia protein trafficking and growth. GPCR overexpression also altered the complement of signaling molecules within the cilia. We found that SSTR3 and type III adenylyl cyclase (ACIII), proteins normally enriched in neuronal cilia, were rarely detected in 5HT6-elongated cilia. Intriguingly, the changes in cilia structure were accompanied by changes in neuronal morphology. -

Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Amy Sue Bogard University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2013 Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Amy Sue Bogard University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Medical Cell Biology Commons, and the Medical Molecular Biology Commons Recommended Citation Bogard, Amy Sue , "Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells" (2013). Theses and Dissertations (ETD). Paper 330. http://dx.doi.org/10.21007/etd.cghs.2013.0029. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Biomedical Sciences Track Molecular Therapeutics and Cell Signaling Research Advisor Rennolds Ostrom, Ph.D. Committee Elizabeth Fitzpatrick, Ph.D. Edwards Park, Ph.D. Steven Tavalin, Ph.D. Christopher Waters, Ph.D. DOI 10.21007/etd.cghs.2013.0029 Comments Six month embargo expired June 2014 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/330 Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells A Dissertation Presented for The Graduate Studies Council The University of Tennessee Health Science Center In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy From The University of Tennessee By Amy Sue Bogard December 2013 Copyright © 2013 by Amy Sue Bogard. -

Oxygenated Fatty Acids Enhance Hematopoiesis Via the Receptor GPR132
Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lahvic, Jamie L. 2017. Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42061504 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132 A dissertation presented by Jamie L. Lahvic to The Division of Medical Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Developmental and Regenerative Biology Harvard University Cambridge, Massachusetts May 2017 © 2017 Jamie L. Lahvic All rights reserved. Dissertation Advisor: Leonard I. Zon Jamie L. Lahvic Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132 Abstract After their specification in early development, hematopoietic stem cells (HSCs) maintain the entire blood system throughout adulthood as well as upon transplantation. The processes of HSC specification, renewal, and homing to the niche are regulated by protein, as well as lipid signaling molecules. A screen for chemical enhancers of marrow transplant in the zebrafish identified the endogenous lipid signaling molecule 11,12-epoxyeicosatrienoic acid (11,12-EET). EET has vasodilatory properties, but had no previously described function on HSCs. -

Dynamic Mass Redistribution Reveals Diverging Importance of PDZ
Pharmacological Research 105 (2016) 13–21 Contents lists available at ScienceDirect Pharmacological Research j ournal homepage: www.elsevier.com/locate/yphrs Dynamic mass redistribution reveals diverging importance of PDZ-ligands for G protein-coupled receptor pharmacodynamics a b b b Nathan D. Camp , Kyung-Soon Lee , Allison Cherry , Jennifer L. Wacker-Mhyre , b b b b Timothy S. Kountz , Ji-Min Park , Dorathy-Ann Harris , Marianne Estrada , b b a b,∗ Aaron Stewart , Nephi Stella , Alejandro Wolf-Yadlin , Chris Hague a Department of Genome Sciences, University of Washington School of Medicine, Seattle, WA 98195, USA b Department of Pharmacology, University of Washington School of Medicine, Seattle, WA 98195, USA a r t i c l e i n f o a b s t r a c t Article history: G protein-coupled receptors (GPCRs) are essential membrane proteins that facilitate cell-to-cell Received 19 October 2015 communication and co-ordinate physiological processes. At least 30 human GPCRs contain a Type I PSD- Received in revised form 95/DLG/Zo-1 (PDZ) ligand in their distal C-terminal domain; this four amino acid motif of X-[S/T]-X-[] 28 December 2015 sequence facilitates interactions with PDZ domain-containing proteins. Because PDZ protein interactions Accepted 1 January 2016 have profound effects on GPCR ligand pharmacology, cellular localization, signal-transduction effector Available online 7 January 2016 coupling and duration of activity, we analyzed the importance of Type I PDZ ligands for the function of 23 full-length and PDZ-ligand truncated (PDZ) human GPCRs in cultured human cells. SNAP-epitope tag Keywords: polyacrylamide gel electrophoresis revealed most Type I PDZ GPCRs exist as both monomers and mul- G protein-coupled receptor timers; removal of the PDZ ligand played minimal role in multimer formation. -

Probing Cell Type–Specific Functions of Gi in Vivo Identifies GPCR Regulators of Insulin Secretion
Probing cell type–specific functions of Gi in vivo identifies GPCR regulators of insulin secretion Jean B. Regard, … , Matthias Hebrok, Shaun R. Coughlin J Clin Invest. 2007;117(12):4034-4043. https://doi.org/10.1172/JCI32994. Research Article Technical Advance Cardiology The in vivo roles of the hundreds of mammalian G protein–coupled receptors (GPCRs) are incompletely understood. To explore these roles, we generated mice expressing the S1 subunit of pertussis toxin, a known inhibitor of Gi/o signaling, under the control of the ROSA26 locus in a Cre recombinase–dependent manner (ROSA26PTX). Crossing ROSA26PTX mice to mice expressing Cre in pancreatic β cells produced offspring with constitutive hyperinsulinemia, increased insulin secretion in response to glucose, and resistance to diet-induced hyperglycemia. This phenotype underscored the known importance of Gi/o and hence of GPCRs for regulating insulin secretion. Accordingly, we quantified mRNA for each of the approximately 373 nonodorant GPCRs in mouse to identify receptors highly expressed in islets and examined the role of several. We report that 3-iodothyronamine, a thyroid hormone metabolite, could negatively and positively regulate insulin secretion via the Gi-coupled α2A-adrenergic receptor and the Gs-coupled receptor Taar1, respectively, and protease- activated receptor–2 could negatively regulate insulin secretion and may contribute to physiological regulation of glucose metabolism. The ROSA26PTX system used in this study represents a new genetic tool to achieve tissue-specific signaling pathway modulation in vivo that can be applied to investigate the role of Gi/o-coupled GPCRs in multiple cell types and processes. Find the latest version: https://jci.me/32994/pdf Technical advance Probing cell type–specific functions of Gi in vivo identifies GPCR regulators of insulin secretion Jean B. -

763.Full.Pdf
1521-0081/70/4/763–835$35.00 https://doi.org/10.1124/pr.117.015388 PHARMACOLOGICAL REVIEWS Pharmacol Rev 70:763–835, October 2018 Copyright © 2018 The Author(s). This is an open access article distributed under the CC BY Attribution 4.0 International license. ASSOCIATE EDITOR: ELIOT H. OHLSTEIN International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature Thomas Günther, Giovanni Tulipano, Pascal Dournaud, Corinne Bousquet, Zsolt Csaba, Hans-Jürgen Kreienkamp, Amelie Lupp, Márta Korbonits, Justo P. Castaño, Hans-Jürgen Wester, Michael Culler,1 Shlomo Melmed, and Stefan Schulz Institute of Pharmacology and Toxicology, Jena University Hospital, Friedrich-Schiller-University, Jena, Germany (T.G., A.L., S.S.); Unit of Pharmacology, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy (G.T.); PROTECT, INSERM, Université Paris Diderot, Sorbonne Paris Cité, Paris, France (P.D., Z.C.); Cancer Research Center of Toulouse, INSERM UMR 1037-University Toulouse III Paul Sabatier, Toulouse, France (C.B.); Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (H.-J.K.); Centre for Endocrinology, William Harvey Research Institute, Barts and London School of Medicine, Queen Mary University of London, London, United Kingdom (M.K.); Maimonides Institute for Biomedical Research of Cordoba, Córdoba, Spain (J.P.C.); Department of Cell Biology, Physiology, and Immunology, University of Córdoba, Córdoba, Spain (J.P. C.); Reina Sofia University Hospital, Córdoba, Spain (J.P.C.); CIBER Fisiopatología de la Obesidad y Nutrición, Córdoba, Spain (J.P.C.); Pharmaceutical Radiochemistry, Technische Universität München, Munich, Germany (H.-J.W.); Culler Consulting LLC, Hopkinton, Massachusetts (M.C.); and Pituitary Center, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, California (S.M.) Downloaded from Abstract ...................................................................................765 I. -

Onadotropin-Releasing Hormone Receptor CLAVULANIC ACID
ARGININE DAPAGLIFLOZIN ORVEPITANT ORLISTAT Nitric oxideNitric-oxide synthase- synthase- inducible brain Sodium/glucose cotransporter 1 Substance-P receptor Gastric triacylglycerolSodium/glucose lipase cotransporter 2 Platelet activating factor receptor Cystic fibrosis transmembrane conductance regulator Pancreatic lipase-related protein 2 Pancreatic triacylglycerol lipase IVACAFTOR LUMACAFTORDENUFOSOL TETRASODIUM Pyrimidinergic receptor P2Y6 GABA receptorPENTOBARBITAL beta-3 subunit TEZOSENTAN Geranylgeranyl transferase type I Endothelin receptor ET-A TIPIFARNIB Leukocyte commonCholecystokinin antigen B receptor PurinergicPyrimidinergic receptor receptor P2Y2 P2Y4 TALNETANT DARUSENTANIRBESARTAN ETOPOSIDECaspase-3HYDROGEN PEROXIDE TETRACYCLINE Transcription factor AP-1 Glutamate racemase REPARIXIN Serotonin (5-HT) receptor Interleukin-8 receptor A ONDANSETRON AZITHROMYCIN Vasopressin V1 receptor Cyclin-dependent kinase 1/cyclin B Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 Smoothened homologNAVITOCLAX SILMITASERTIB VASOPRESSIN AMSACRINEDNA topoisomerase II IVERMECTINalpha EPIRUBICINAMILORIDE HYDROCHLORIDE HYDROCHLORIDE VISMODEGIBBcl2-antagonist of cell death (BAD) MIDOSTAURIN CANNABIDIOL ICLAPRIM CLARITHROMYCIN IRINOTECAN HYDROCHLORIDE HYDRATE VINCRISTINELEUCOVORIN SULFATE CALCIUM DNA topoisomerase II TELITHROMYCINCARBOPLATIN CEFDITOREN PIVOXIL BENFOTIAMINETERIPARATIDE RIFAMYCIN RIFAXIMIN GLUTATHIONE BENZALKONIUM CHLORIDE Mammalian target of Rapamycin (mTORC1) CLINDAMYCIN BETAMETHASONEDONEPEZILMEPARTRICIN HYDROCHLORIDE RUTIN c-Jun -

Characterisation of L-Cell Secretory Mechanisms and Colonic
Characterisation of L -cell secretory mechanisms and colonic enteroendocrine cell subpopulations Lawrence Billing Downing College, Cambridge This dissertation is submitted for the degree of Doctor of Philosophy September 2018 Abstract Enteroendocrine cells (EECs) are chemosensitive cells of the gastrointestinal epithelium that exert a wide range of physiological effects via production and secretion of hormones in response to ingested nutrients, bacterial metabolites and systemic signals. Glucagon-like peptide-1 (GLP-1) is one such hormone secreted from so-called L-cells found in both the small and large intestines. GLP-1 exerts an anorexigenic effect and together with glucose- dependent insulinotropic polypeptide (GIP), restores postprandial normoglycaemia through the incretin effect. These effects are exploited by GLP-1 analogues in the treatment of type 2 diabetes. GLP-1 may also contribute to weight-loss and remission of type 2 diabetes following bariatric surgery which increases postprandial GLP-1 excursions. Here we investigated stimulus secretion coupling in L-cells. A novel 2D culture system from murine small intestinal organoids was established as an in vitro model. This was used to characterise synergistic stimulation of GLP-1 secretion in response to concomitant stimulation by bile acids through the Gs-protein coupled receptor GPBAR1 and free fatty acids through the Gq-coupled receptor FFAR1. Roughly half of colonic, but not small intestinal, L-cells co-produce the orexigenic peptide insulin-like peptide 5 (INSL5). This hitherto poorly examined subpopulation of L-cells was characterised through transcriptomic analysis, intracellular calcium imaging (using a novel GCaMP6F-based transgenic mouse model), LC/MS peptide quantification and 3D super resolution microscopy (3D-SIM). -

RT² Profiler PCR Array (384-Well Format) Mouse G Protein Coupled Receptors 384HT
RT² Profiler PCR Array (384-Well Format) Mouse G Protein Coupled Receptors 384HT Cat. no. 330231 PAMM-3009ZE For pathway expression analysis Format For use with the following real-time cyclers RT² Profiler PCR Array, Applied Biosystems® models 7900HT (384-well block), Format E ViiA™ 7 (384-well block); Bio-Rad CFX384™ RT² Profiler PCR Array, Roche® LightCycler® 480 (384-well block) Format G Description The Mouse G Protein Coupled Receptors 384HT RT² Profiler™ PCR Array profiles the expression of a comprehensive panel of 370 genes encoding the most important G Protein Coupled Receptors (GPCR). GPCR regulate a number of normal biological processes and play roles in the pathophysiology of many diseases upon dysregulation of their downstream signal transduction activities. As a result, they represent 30 percent of the targets for all current drug development. Developing drug screening assays requires a survey of which GPCR the chosen cell-based model system expresses, to determine not only the expression of the target GPCR, but also related GPCR to assess off-target side effects. Expression of other unrelated GPCR (even orphan receptors whose ligand are unknown) may also correlate with off-target side effects. The ligands that bind and activate the receptors on this array include neurotransmitters and neuropeptides, hormones, chemokines and cytokines, lipid signaling molecules, light-sensitive compounds, and odorants and pheromones. The normal biological processes regulated by GPCR include, but are not limited to, behavioral and mood regulation (serotonin, dopamine, GABA, glutamate, and other neurotransmitter receptors), autonomic (sympathetic and parasympathetic) nervous system transmission (blood pressure, heart rate, and digestive processes via hormone receptors), inflammation and immune system regulation (chemokine receptors, histamine receptors), vision (opsins like rhodopsin), and smell (olfactory receptors for odorants and vomeronasal receptors for pheromones). -

SSTR3 Is a Putative Target for the Medical Treatment of Gonadotroph Adenomas of the Pituitary
M Lee et al. Somatostatin receptors in 22:1 111–119 Research gonadotroph adenomas SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary Misu Lee, Amelie Lupp1, Nigel Mendoza2, Niamh Martin3, Rudi Beschorner4, Ju¨rgen Honegger5,Ju¨rgen Schlegel6, Talia Shively3, Elke Pulz, Stefan Schulz1, Federico Roncaroli3 and Natalia S Pellegata Correspondence Institute of Pathology, Helmholtz Zentrum Mu¨ nchen, D-85764 Neuherberg, Germany should be addressed 1Department of Pharmacology and Toxicology, Jena University Hospital, Friedrich Schiller University, Jena, Germany to N S Pellegata; F Roncaroli 2Departments of Neurosurgery 3Medicine, Imperial College, St Dunstans Road, London W6 8RP, UK Emails 4Department of Neuropathology, Institute for Pathology and Neuropathology 5Department of Neurosurgery, natalia.pellegata@ University of Tu¨ bingen, Tu¨ bingen, Germany helmholtz-muenchen.de; 6Institute of Pathology, Technical University of Munich, Mu¨ nchen, Germany [email protected] Abstract Gonadotroph pituitary adenomas (GPAs) often present as invasive macroadenomas not Key Words amenable to complete surgical resection. Radiotherapy is the only post-operative option for " somatostatin receptors patients with large invasive or recurrent lesions. No medical treatment is available for these " gonadotroph adenoma patients. The somatostatin analogs (SSAs) octreotide and lanreotide that preferentially " aggressive adenoma target somatostatin receptor type 2 (SSTR2) have little effect on GPAs. It is widely accepted " pasireotide that the expression of specific SSTR subtypes determines the response to SSAs. Given that Endocrine-Related Cancer previous studies on mRNA and protein expression of SSTRs in GPAs have generated conflicting results, we investigated the expression of SSTR2, SSTR3, and SSTR5 (the main targets of available SSAs) in a clinically and pathologically well-characterized cohort of 108 patients with GPAs. -

Somatostatin Receptor Subtype Expression in the Human Heart: Differential Expression by Myocytes and fibroblasts
379 Somatostatin receptor subtype expression in the human heart: differential expression by myocytes and fibroblasts William H T Smith, R Unnikrishnan Nair1, Dawn Adamson2, Mark T Kearney3, Stephen G Ball and Anthony J Balmforth Integrated Molecular Cardiology Group, University of Leeds, Leeds LS2 9JT, UK 1Department of Cardiothoracic Surgery, The General Infirmary at Leeds, Leeds LS1 3EX, UK 2National Heart and Lung Institute, Imperial College, London SW3 6LY, UK 3GKT School of Medicine, London WC2R 2LS, UK (Requests for offprints should be addressed to A J Balmforth; Email: [email protected]) Abstract In acromegaly, somatostatin receptor ligands (SRLs) can and sst5) were shown to be co-expressed by the human ameliorate left ventricular hypertrophy (LVH) and their heart. These receptors were present in both atrial and use is associated with demonstrable improvements in ventricular tissue. Human cardiac myocytes expressed various parameters of cardiac function. It remains unclear mRNA for only sst1 and sst2, while human cardiac as to whether these beneficial effects are principally fibroblasts expressed all four subtypes found in whole heart attributable to falling GH and IGF-I levels, or whether tissue. The expression of functional somatostatin receptors SRLs exert independent direct effects on the heart via on human cardiac fibroblasts was confirmed by mobilis- somatostatin receptors. To help address this issue, we have ation of intracellular calcium in response to somatostatin. sought to investigate somatostatin receptor expression The presence of cardiac somatostatin receptors raises the in human heart. A human heart cDNA library was possibility of a direct effect of somatostatin analogues on probed using PCR techniques to determine expression of the heart.