SSTR3 Is a Putative Target for the Medical Treatment of Gonadotroph Adenomas of the Pituitary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Drug-Like Somatostatin Receptor 4 Agonists Are Potential Analgesics for Neuropathic Pain
International Journal of Molecular Sciences Article Novel Drug-Like Somatostatin Receptor 4 Agonists are Potential Analgesics for Neuropathic Pain 1,2, 3, 1,2 4 1,2 Boglárka Kántás y, Rita Börzsei y, Éva Sz˝oke ,Péter Bánhegyi , Ádám Horváth , 1,2 1,2 1 1,2, Ágnes Hunyady , Éva Borbély , Csaba Hetényi , Erika Pintér y and 1,2, , Zsuzsanna Helyes * y 1 Department of Pharmacology and Pharmacotherapy, Medical School, University of Pécs, Szigeti str. 12, H-7624 Pécs, Hungary 2 Szentágothai Research Centre and Centre for Neuroscience, University of Pécs, Ifjúság str. 20, H-7624 Pécs, Hungary 3 Department of Pharmacology, Faculty of Pharmacy, University of Pécs, Szigeti str. 12, H-7624 Pécs, Hungary 4 Avicor Ltd., Herman Ottó str. 15, H-1022 Budapest, Hungary * Correspondence: [email protected] These authors contributed equally to this work. y Received: 14 October 2019; Accepted: 9 December 2019; Published: 11 December 2019 Abstract: Somatostatin released from the capsaicin-sensitive sensory nerves mediates analgesic and anti-inflammatory effects via the somatostatin sst4 receptor without endocrine actions. Therefore, sst4 is considered to be a novel target for drug development in pain including chronic neuropathy, which is an emerging unmet medical need. Here, we examined the in silico binding, the sst4-linked G-protein activation on stable receptor expressing cells (1 nM to 10 µM), and the effects of our novel pyrrolo-pyrimidine molecules in mouse inflammatory and neuropathic pain models. All four of the tested compounds (C1–C4) bind to the same binding site of the sst4 receptor with similar interaction energy to high-affinity reference sst4 agonists, and they all induce G-protein activation. -

Procedure Guideline for Somatostatin Receptor Scintigraphy with 111In-Pentetreotide
PROCEDURE GUIDELINES Procedure Guideline for Somatostatin Receptor Scintigraphy with 111In-Pentetreotide Helena R. Balon, Stanley J. Goldsmith, Barry A. Siegel, Edward B. Silberstein, Eric P. Krenning, Otto Lang, and Kevin J. Donohoe William Beaumont Hospital, Royal Oak, Michigan; New York Hospital–Cornell Medical, New York, NY; Mallinckrodt Institute of Radiology, St. Louis, Missouri; University of Cincinnati Medical Center, Cincinnati, Ohio; Beth Israel Deaconess Medical Center, Boston, Massachusetts; University Hospital Dijkzigt, Rotterdam, The Netherlands; and Third Medical School, Charles University, Prague, Czech Republic ● Paraganglioma. Key Words: guideline; octreotide; somatostatin receptor ● Pituitary adenomas. J Nucl Med 2001; 42:1134–1138 ● Small cell lung carcinoma. Other tumors and disease processes may also be detected by 111In-pentetreotide scintigraphy and knowledge of the PART I: PURPOSE patient’s history is thus important. These disorders may The purpose of this guideline is to assist nuclear medicine include, but are not limited to, the following: practitioners in recommending, performing, interpreting, and reporting the results of somatostatin receptor scintigra- ● Astrocytomas. phy with 111In-pentetreotide. ● Benign and malignant bone tumors. ● Breast carcinoma. PART II: BACKGROUND INFORMATION AND ● Differentiated thyroid carcinoma (papillary, follicular, DEFINITIONS and Hu¨rthle cell). 111In-pentetreotide is a [111In-DTPA-D-Phe-] conjugate of ● Lymphoma (Hodgkin’s and non-Hodgkin’s). octreotide, a somatostatin analog -

Coexpression of Human Somatostatin Receptor-2 (SSTR2) and SSTR3 Modulates Antiproliferative Signaling and Apoptosis Sajad a War and Ujendra Kumar*
War and Kumar Journal of Molecular Signaling 2012, 7:5 http://www.thrombosisjournal.com/7/1/5 RESEARCH ARTICLE Open Access Coexpression of human somatostatin receptor-2 (SSTR2) and SSTR3 modulates antiproliferative signaling and apoptosis Sajad A War and Ujendra Kumar* Abstract Background: Somatostatin (SST) via five Gi coupled receptors namely SSTR1-5 is known to inhibit cell proliferation by cytostatic and cytotoxic mechanisms. Heterodimerization plays a crucial role in modulating the signal transduction pathways of SSTR subtypes. In the present study, we investigated human SSTR2/SSTR3 heterodimerization, internalization, MAPK signaling, cell proliferation and apoptosis in HEK-293 cells in response to SST and specific agonists for SSTR2 and SSTR3. Results: Although in basal conditions, SSTR2 and SSTR3 colocalize at the plasma membrane and exhibit heterodimerization, the cell surface distribution of both receptors decreased upon agonist activation and was accompanied by a parallel increase in intracellular colocalization. Receptors activation by SST and specific agonists significantly decreased cAMP levels in cotransfected cells in comparison to control. Agonist-mediated modulation of pERK1/2 was time and concentration-dependent, and pronounced in serum-deprived conditions. pERK1/2 was inhibited in response to SST; conversely receptor-specific agonist treatment caused inhibition at lower concentration and activation at higher concentration. Strikingly, ERK1/2 phosphorylation was sustained upon prolonged treatment with SST but not with receptor-specific agonists. On the other hand, SST and receptor-specific agonists modulated p38 phosphorylation time-dependently. The receptor activation in cotransfected cells exhibits Gi-dependent inhibition of cell proliferation attributed to increased PARP-1 expression and TUNEL staining, whereas induction of p21 and p27Kip1 suggests a cytostatic effect. -

Somatostatin Receptor Type 2A Immunohistochemistry in Neuroendocrine Tumors: a Proposal of Scoring System Correlated with Somatostatin Receptor Scintigraphy
Modern Pathology (2007) 20, 1172–1182 & 2007 USCAP, Inc All rights reserved 0893-3952/07 $30.00 www.modernpathology.org Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy Marco Volante1, Maria Pia Brizzi1, Antongiulio Faggiano2, Stefano La Rosa3, Ida Rapa1, Anna Ferrero1, Gelsomina Mansueto4, Luisella Righi1, Silvana Garancini5, Carlo Capella3, Gaetano De Rosa4, Luigi Dogliotti1, Annamaria Colao2 and Mauro Papotti1 1Department of Clinical and Biological Sciences, San Luigi Hospital, University of Turin, Orbassano, Turin, Italy; 2Department of Molecular and Clinical Endocrinology and Oncology, ‘Federico II’ University, Naples, Italy; 3Section of Anatomic Pathology, Department of Human Morphology, University of Insubria and Ospedale di Circolo, Varese, Italy; 4Department of General Pathology, Medicine, Human Pathology and Clinical Pathology, University of Naples ‘Federico II’, Naples, Italy and 5Department of Nuclear Medicine, Ospedale di Circolo, Varese, Italy Typing somatostatin receptor expression in neuroendocrine tumors is of relevance to target somatostatin analogue-based diagnostic approach and treatment. The expanding use of immunohistochemistry to detect somatostatin receptors is to date not paralleled by an accurate methodological setting and standardized interpretation of the results. A multicentric study was designed to compare somatostatin receptor immunohistochemical expression with in vivo scintigraphic data and verify its usefulness in the clinical management of neuroendocrine tumors. After methodological setting by testing different somatostatin receptor antibodies, 107 cases of neuroendocrine tumors with available somatostatin receptor scintigraphy data and pathological material were retrospectively analyzed for somatostatin receptor types 2A, 3 and 5 immunohis- tochemical expression, and compared with scintigraphic images and, whenever available, with the clinical response to somatostatin analogue treatment. -

Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors
Journal of Nuclear Medicine, published on September 4, 2014 as doi:10.2967/jnumed.114.142000 CONTINUING EDUCATION Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors Clément Morgat1–3, Anil Kumar Mishra2–4, Raunak Varshney4, Michèle Allard1,2,5, Philippe Fernandez1–3, and Elif Hindié1–3 1CHU de Bordeaux, Service de Médecine Nucléaire, Bordeaux, France; 2University of Bordeaux, INCIA, UMR 5287, Talence, France; 3CNRS, INCIA, UMR 5287, Talence, France; 4Division of Cyclotron and Radiopharmaceutical Sciences, Institute of Nuclear Medicine and Allied Sciences, DRDO, New Delhi, India; and 5EPHE, Bordeaux, France Learning Objectives: On successful completion of this activity, participants should be able to list and discuss (1) the presence of bombesin receptors, neurotensin receptors, or neuropeptide-Y receptors in some major tumors; (2) the perspectives offered by radiolabeled peptides targeting these receptors for imaging and therapy; and (3) the choice between agonists and antagonists for tumor targeting and the relevance of various PET radionuclides for molecular imaging. Financial Disclosure: The authors of this article have indicated no relevant relationships that could be perceived as a real or apparent conflict of interest. CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through October 2017. -

Identification of Neuropeptide Receptors Expressed By
RESEARCH ARTICLE Identification of Neuropeptide Receptors Expressed by Melanin-Concentrating Hormone Neurons Gregory S. Parks,1,2 Lien Wang,1 Zhiwei Wang,1 and Olivier Civelli1,2,3* 1Department of Pharmacology, University of California Irvine, Irvine, California 92697 2Department of Developmental and Cell Biology, University of California Irvine, Irvine, California 92697 3Department of Pharmaceutical Sciences, University of California Irvine, Irvine, California 92697 ABSTRACT the MCH system or demonstrated high expression lev- Melanin-concentrating hormone (MCH) is a 19-amino- els in the LH and ZI, were tested to determine whether acid cyclic neuropeptide that acts in rodents via the they are expressed by MCH neurons. Overall, 11 neuro- MCH receptor 1 (MCHR1) to regulate a wide variety of peptide receptors were found to exhibit significant physiological functions. MCH is produced by a distinct colocalization with MCH neurons: nociceptin/orphanin population of neurons located in the lateral hypothala- FQ opioid receptor (NOP), MCHR1, both orexin recep- mus (LH) and zona incerta (ZI), but MCHR1 mRNA is tors (ORX), somatostatin receptors 1 and 2 (SSTR1, widely expressed throughout the brain. The physiologi- SSTR2), kisspeptin recepotor (KissR1), neurotensin cal responses and behaviors regulated by the MCH sys- receptor 1 (NTSR1), neuropeptide S receptor (NPSR), tem have been investigated, but less is known about cholecystokinin receptor A (CCKAR), and the j-opioid how MCH neurons are regulated. The effects of most receptor (KOR). Among these receptors, six have never classical neurotransmitters on MCH neurons have been before been linked to the MCH system. Surprisingly, studied, but those of most neuropeptides are poorly several receptors thought to regulate MCH neurons dis- understood. -

Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms
Review Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms Melpomeni Fani 1,*, Petra Kolenc Peitl 2 and Irina Velikyan 3 1 Division of Radiopharmaceutical Chemistry, University Hospital of Basel, 4031 Basel, Switzerland; [email protected] 2 Department of Nuclear Medicine, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia; [email protected] 3 Department of Medicinal Chemistry, Uppsala University, 751 23 Uppsala, Sweden; [email protected] * Correspondence: [email protected]; Tel.: +41-61-556-58-91; Fax: +41-61-265-49-25 Academic Editor: Klaus Kopka Received: 7 February 2017; Accepted: 9 March 2017; Published: 15 March 2017 Abstract: Nuclear medicine plays a pivotal role in the management of patients affected by neuroendocrine neoplasms (NENs). Radiolabeled somatostatin receptor analogs are by far the most advanced radiopharmaceuticals for diagnosis and therapy (radiotheranostics) of NENs. Their clinical success emerged receptor-targeted radiolabeled peptides as an important class of radiopharmaceuticals and it paved the way for the investigation of other radioligand-receptor systems. Besides the somatostatin receptors (sstr), other receptors have also been linked to NENs and quite a number of potential radiolabeled peptides have been derived from them. The Glucagon- Like Peptide-1 Receptor (GLP-1R) is highly expressed in benign insulinomas, the Cholecystokinin 2 (CCK2)/Gastrin receptor is expressed in different NENs, in particular medullary thyroid cancer, and the Glucose-dependent Insulinotropic Polypeptide (GIP) receptor was found to be expressed in gastrointestinal and bronchial NENs, where interestingly, it is present in most of the sstr-negative and GLP-1R-negative NENs. Also in the field of sstr targeting new discoveries brought into light an alternative approach with the use of radiolabeled somatostatin receptor antagonists, instead of the clinically used agonists. -

Development of a Universal High-Throughput Calcium Assay for G-Protein-Coupled Receptors with Promiscuous G-Protein Gα15/16
Acta Pharmacol Sin 2008 Apr; 29 (4): 507–516 Full-length article Development of a universal high-throughput calcium assay for G-pro- tein-coupled receptors with promiscuous G-protein Gα15/161 Ting ZHU2,4, Li-yan FANG2,3, Xin XIE2,3,5 2The National Center for Drug Screening, 3Shanghai Institute of Materia Medica, 4Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 201203, China Key words Abstract G-protein-coupled receptors; G-protein; Aim: To develop a universal high-throughput screening assay based on Gα15/16- Gα15/16; high-throughput screening; mediated calcium mobilization for the identification of novel modulators of G- calcium assay; GTPγS binding protein-coupled receptors (GPCR). Methods: In the present study, CHO-K1 or 1Project supported by grants from the Chinese HEK293 cells were co-transfected with plasmids encoding promiscuous G-protein Academy of Sciences (No KSCX2-YW-R- Gα15/16 and various receptors originally coupled to Gαs, Gαi, or Gαq pathways. 18), the Ministry of Science and Technology of China (No 2006AA020602), and the Intracellular calcium change was monitored with fluorescent dye Fluo-4. Results: Shanghai Commission of Science and We found out for all the receptors tested, Gα15/16 could shift the receptors’ Technology (No 05PJ14313, 06DZ22907). coupling to the calcium mobilization pathway, and the EC50 values of the ligands 5Correspondence to Dr Xin XIE. Phn 86-21-5080-1313, ext 156. generated with this method were comparable with reported values that were ob- Fax 86-21-5080-0721. tained using traditional methods. This assay was validated and optimized with E-mail [email protected] the δ-opioid receptor, which originally coupled to Gαi and was recently found to play important roles in neurodegenerative and autoimmune diseases. -

26985.Full.Pdf
Regulating quantal size of neurotransmitter release through a GPCR voltage sensor Quanfeng Zhanga,1, Bing Liua,1, Yinglin Lia,1, Lili Yina, Muhammad Younusa, Xiaohan Jianga, Zhaohan Lina, Xiaoxuan Suna, Rong Huanga, Bin Liua, Qihui Wua, Feipeng Zhua, and Zhuan Zhoua,2 aState Key Laboratory of Membrane Biology and Beijing Key Laboratory of Cardiometabolic Molecular Medicine, Institute of Molecular Medicine and Peking-Tsinghua Center for Life Sciences and PKU-IDG/McGovern Institute for Brain Research, Peking University, 100871 Beijing, China Edited by Robert H. Edwards, University of California, San Francisco, CA, and approved September 11, 2020 (received for review March 25, 2020) Current models emphasize that membrane voltage (Vm) depolarization- ATP is the ligand of two families of purinergic receptors, P2Xs induced Ca2+ influx triggers the fusion of vesicles to the plasma and P2Ys. P2Xs are ion channels and P2Ys are GPCRs, in- membrane. In sympathetic adrenal chromaffin cells, activation of cluding Gq (P2Y1, 2, 4, 6, 11) and Gi types (P2Y12, 13, 14) (27, 28). a variety of G protein coupled receptors (GPCRs) can inhibit quantal Among the P2Ys, P2Y1 exists in most tissues, including epithelial size (QS) through the direct interaction of G protein Giβγ subunits and endothelial cells, platelets, and immune cells (27, 29), and with exocytosis fusion proteins. Here we report that, independently P2Y12 is strongly expressed on platelets, where it plays funda- from Ca2+, Vm (action potential) per se regulates the amount of mental roles in their activation and aggregation (28, 30, 31), as catecholamine released from each vesicle, the QS. The Vm regula- well as in microglia (32, 33), smooth muscle cells (34), and tion of QS was through ATP-activated GPCR-P2Y12 receptors. -
PDF (Appendices)
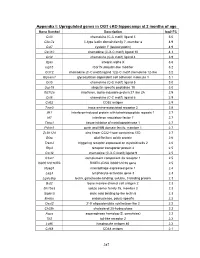
Appendix I: Upregulated genes in OGT cKO hippocampi at 2 months of age Gene Symbol Description log2 FC Ccl3 chemokine (C-C motif) ligand 3 5.0 Clec7a C-type lectin domain family 7, member a 4.9 Cst7 cystatin F (leukocystatin) 4.9 Cxcl10 chemokine (C-X-C motif) ligand 10 4.3 Ccl4 chemokine (C-C motif) ligand 4 3.9 Itgax integrin alpha X 3.6 Isg15 ISG15 ubiquitin-like modifier 3.2 Ccl12 chemokine (C-C motif) ligand 12|c-C motif chemokine 12-like 3.2 Glycam1 glycosylation dependent cell adhesion molecule 1 3.1 Ccl5 chemokine (C-C motif) ligand 5 3.0 Usp18 ubiquitin specific peptidase 18 3.0 Ifi27l2a interferon, alpha-inducible protein 27 like 2A 2.9 Ccl6 chemokine (C-C motif) ligand 6 2.9 Cd52 CD52 antigen 2.9 Taar3 trace amine-associated receptor 3 2.8 Ifit1 interferon-induced protein with tetratricopeptide repeats 1 2.7 Irf7 interferon regulatory factor 7 2.7 Timp1 tissue inhibitor of metalloproteinase 1 2.7 Pyhin1 pyrin and HIN domain family, member 1 2.7 Zc3h12d zinc finger CCCH type containing 12D 2.7 Gfap glial fibrillary acidic protein 2.6 Trem2 triggering receptor expressed on myeloid cells 2 2.6 Rtp4 receptor transporter protein 4 2.5 Cxcl9 chemokine (C-X-C motif) ligand 9 2.5 C3ar1 complement component 3a receptor 1 2.5 I830012O16Rik RIKEN cDNA I830012O16 gene 2.5 Mpeg1 macrophage expressed gene 1 2.4 Lag3 lymphocyte-activation gene 3 2.3 Lgals3bp lectin, galactoside-binding, soluble, 3 binding protein 2.3 Bst2 bone marrow stromal cell antigen 2 2.3 Slc15a3 solute carrier family 15, member 3 2.3 Siglec5 sialic acid binding Ig-like lectin 5 2.3 Endou endonuclease, polyU-specific 2.2 Oasl2 2'-5' oligoadenylate synthetase-like 2 2.2 Ch25h cholesterol 25-hydroxylase 2.2 Aspg asparaginase homolog (S. -

Arborization of Dendrites by Developing Neocortical Neurons Is Dependent on Primary Cilia and Type 3 Adenylyl Cyclase
2626 • The Journal of Neuroscience, February 6, 2013 • 33(6):2626–2638 Development/Plasticity/Repair Arborization of Dendrites by Developing Neocortical Neurons Is Dependent on Primary Cilia and Type 3 Adenylyl Cyclase Sarah M. Guadiana,1 Susan Semple-Rowland,1 Daniel Daroszewski,1 Irina Madorsky,1 Joshua J. Breunig,2 Kirk Mykytyn,3 and Matthew R. Sarkisian1 1Department of Neuroscience, McKnight Brain Institute, University of Florida, Gainesville, Florida 32610-0244, 2Cedars-Sinai Regenerative Medicine Institute, Los Angeles, California 90036, and 3Department of Pharmacology, Wexner Medical Center, Ohio State University, Columbus, Ohio 43210 The formation of primary cilia is a highly choreographed process that can be disrupted in developing neurons by overexpressing neuromodulatory G-protein-coupled receptors GPCRs or by blocking intraflagellar transport. Here, we examined the effects of overex- pressing the ciliary GPCRs, 5HT6 and SSTR3, on cilia structure and the differentiation of neocortical neurons. Neuronal overexpression of 5HT6 and SSTR3 was achieved by electroporating mouse embryo cortex in utero with vectors encoding these receptors. We found that overexpression of ciliary GPCRs in cortical neurons, especially 5HT6, induced the formation of long (Ͼ30 m) and often forked cilia. These changes were associated with increased levels of intraflagellar transport proteins and accelerated ciliogenesis in neonatal neocor- tex, the induction of which required Kif3a, an anterograde motor critical for cilia protein trafficking and growth. GPCR overexpression also altered the complement of signaling molecules within the cilia. We found that SSTR3 and type III adenylyl cyclase (ACIII), proteins normally enriched in neuronal cilia, were rarely detected in 5HT6-elongated cilia. Intriguingly, the changes in cilia structure were accompanied by changes in neuronal morphology. -

Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Amy Sue Bogard University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2013 Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Amy Sue Bogard University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Medical Cell Biology Commons, and the Medical Molecular Biology Commons Recommended Citation Bogard, Amy Sue , "Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells" (2013). Theses and Dissertations (ETD). Paper 330. http://dx.doi.org/10.21007/etd.cghs.2013.0029. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Biomedical Sciences Track Molecular Therapeutics and Cell Signaling Research Advisor Rennolds Ostrom, Ph.D. Committee Elizabeth Fitzpatrick, Ph.D. Edwards Park, Ph.D. Steven Tavalin, Ph.D. Christopher Waters, Ph.D. DOI 10.21007/etd.cghs.2013.0029 Comments Six month embargo expired June 2014 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/330 Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells A Dissertation Presented for The Graduate Studies Council The University of Tennessee Health Science Center In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy From The University of Tennessee By Amy Sue Bogard December 2013 Copyright © 2013 by Amy Sue Bogard.