Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corticotropin-Releasing Hormone Physiology
European Journal of Endocrinology (2006) 155 S71–S76 ISSN 0804-4643 Corticotropin-releasing hormone physiology Joseph A Majzoub Division of Endocrinology, Children’s Hospital Boston, Thomas Morgan Rotch Professor of Pediatrics, Harvard Medical School, 300 Longwood Avenue, Boston, Massachusetts 02115, USA (Correspondence should be addressed to J A Majzoub; Email: [email protected]) Abstract Corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor, is a highly conserved peptide hormone comprising 41 amino acid residues. Its name derives from its role in the anterior pituitary, where it mediates the release of corticotropin (ACTH) leading to the release of adrenocortical steroids. CRH is the major hypothalamic activator of the hypothalamic–pituitary– adrenal (HPA)axis. Major functions of the HPAinclude: (i) influencing fetal development of major organ systems including lung, liver, and gut, (ii) metabolic functions, including the maintenance of normal blood glucose levels during the fasting state via glycogenolysis and gluconeogenesis, (iii) modulation of immune function, and (iv) maintenance of cardiovascular tone. In addition, CRH, acting both directly and via the HPA, has a role in regulating several neuroendocrine functions including behavior, food intake, reproduction, growth, immune function, and autonomic function. CRH has been localized to the paraventricular nucleus (PVN) of the hypothalamus, which projects to the median eminence and other hypothalamic and midbrain targets. The CRH gene is composed of two exons. The CRH promoter contains a cAMP-response element, and the intron contains a restrictive element-1/neuron restrictive silencing element (RE-1/NRSE) sequence. Recently, a family of CRH-related peptides, termed the urocortins, has been identified. -

Novel Drug-Like Somatostatin Receptor 4 Agonists Are Potential Analgesics for Neuropathic Pain
International Journal of Molecular Sciences Article Novel Drug-Like Somatostatin Receptor 4 Agonists are Potential Analgesics for Neuropathic Pain 1,2, 3, 1,2 4 1,2 Boglárka Kántás y, Rita Börzsei y, Éva Sz˝oke ,Péter Bánhegyi , Ádám Horváth , 1,2 1,2 1 1,2, Ágnes Hunyady , Éva Borbély , Csaba Hetényi , Erika Pintér y and 1,2, , Zsuzsanna Helyes * y 1 Department of Pharmacology and Pharmacotherapy, Medical School, University of Pécs, Szigeti str. 12, H-7624 Pécs, Hungary 2 Szentágothai Research Centre and Centre for Neuroscience, University of Pécs, Ifjúság str. 20, H-7624 Pécs, Hungary 3 Department of Pharmacology, Faculty of Pharmacy, University of Pécs, Szigeti str. 12, H-7624 Pécs, Hungary 4 Avicor Ltd., Herman Ottó str. 15, H-1022 Budapest, Hungary * Correspondence: [email protected] These authors contributed equally to this work. y Received: 14 October 2019; Accepted: 9 December 2019; Published: 11 December 2019 Abstract: Somatostatin released from the capsaicin-sensitive sensory nerves mediates analgesic and anti-inflammatory effects via the somatostatin sst4 receptor without endocrine actions. Therefore, sst4 is considered to be a novel target for drug development in pain including chronic neuropathy, which is an emerging unmet medical need. Here, we examined the in silico binding, the sst4-linked G-protein activation on stable receptor expressing cells (1 nM to 10 µM), and the effects of our novel pyrrolo-pyrimidine molecules in mouse inflammatory and neuropathic pain models. All four of the tested compounds (C1–C4) bind to the same binding site of the sst4 receptor with similar interaction energy to high-affinity reference sst4 agonists, and they all induce G-protein activation. -

Expression of Urocortin and Corticotropin-Releasing Hormone Receptors in the Horse Thyroid Gland
Cell Tissue Res DOI 10.1007/s00441-012-1450-4 REGULAR ARTICLE Expression of urocortin and corticotropin-releasing hormone receptors in the horse thyroid gland Caterina Squillacioti & Adriana De Luca & Sabrina Alì & Salvatore Paino & Giovanna Liguori & Nicola Mirabella Received: 11 January 2012 /Accepted: 3 May 2012 # Springer-Verlag 2012 Abstract Urocortin (UCN) is a 40-amino-acid peptide and UCN, CRHR1 and CRHR2 and that UCN plays a role in a member of the corticotropin-releasing hormone (CRH) the regulation of thyroid physiological functions through a family, which includes CRH, urotensin I, sauvagine, paracrine mechanism. UCN2 and UCN3. The biological actions of CRH family peptides are mediated via two types of G-protein-coupled Keywords Follicular cells . C-cells . RT-PCR . receptors, namely CRH type 1 receptor (CRHR1) and CRH Immunohistochemistry . Horse type 2 receptor (CRHR2). The biological effects of these peptides are mediated and modulated not only by CRH receptors but also via a highly conserved CRH-binding Introduction protein (CRHBP). Our aim was to investigate the expression of UCN, CRHR1, CRHR2 and CRHBP by immunohisto- Urocortin (UCN) is a peptide of 40 amino acids and is a chemistry, Western blot and reverse transcription with the member of the corticotropin-releasing hormone (CRH) fam- polymerase chain reaction (RT-PCR) in the horse thyroid ily, which includes CRH, urotensin I, sauvagine, UCN2 and gland. The results showed that UCN, CRHR1 and CRHR2 UCN3. Vaughan et al. (1995) were the first to identify UCN, were expressed in the thyroid gland, whereas CRHBP was which exhibits 45% homology to CRH (Latchman 2002; not expressed. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -
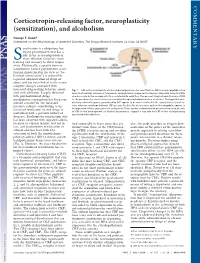
Corticotropin-Releasing Factor, Neuroplasticity (Sensitization), and Alcoholism
COMMENTARY Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism George F. Koob* Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA 92037 ensitization is a ubiquitous bio- logical phenomenon that has a role in the neuroadaptation of many different functions, from Slearning and memory to stress respon- sivity. Historically, a specific form of sensitization termed psychomotor sensi- tization (mistermed in my view as ‘‘be- havioral sensitization’’) is induced by repeated administration of drugs of abuse and has been linked to the neuro- adaptive changes associated with increased drug-seeking behavior associ- Fig. 1. CNS actions relevant to alcohol-induced psychomotor sensitization. CRF is a neuropeptide in the ated with addiction. Largely observed brain that controls autonomic, hormonal, and behavioral responses to stressors. New data show that CRF with psychostimulant drugs, also has a role in the neuroplasticity associated with addiction. The present study extends the role of CRF psychomotor sensitization has been con- to the psychomotor sensitization associated with repeated administration of alcohol. The hypothalamic- sidered a model for the increased pituitary-adrenal responses produced by CRF appear to be more involved in the acquisition of sensitiza- incentive salience contributing to the tion, whereas extrahypothalamic CRF systems, likely to be in structures such as the amygdala, appear to increased motivation to seek drugs in be important for the expression of sensitization. These results, combined with previous studies on the role of CRF in the development of alcohol dependence, suggest a key role for CRF in the neuroplasticity individuals with a previous history of associated with addiction. -

Procedure Guideline for Somatostatin Receptor Scintigraphy with 111In-Pentetreotide
PROCEDURE GUIDELINES Procedure Guideline for Somatostatin Receptor Scintigraphy with 111In-Pentetreotide Helena R. Balon, Stanley J. Goldsmith, Barry A. Siegel, Edward B. Silberstein, Eric P. Krenning, Otto Lang, and Kevin J. Donohoe William Beaumont Hospital, Royal Oak, Michigan; New York Hospital–Cornell Medical, New York, NY; Mallinckrodt Institute of Radiology, St. Louis, Missouri; University of Cincinnati Medical Center, Cincinnati, Ohio; Beth Israel Deaconess Medical Center, Boston, Massachusetts; University Hospital Dijkzigt, Rotterdam, The Netherlands; and Third Medical School, Charles University, Prague, Czech Republic ● Paraganglioma. Key Words: guideline; octreotide; somatostatin receptor ● Pituitary adenomas. J Nucl Med 2001; 42:1134–1138 ● Small cell lung carcinoma. Other tumors and disease processes may also be detected by 111In-pentetreotide scintigraphy and knowledge of the PART I: PURPOSE patient’s history is thus important. These disorders may The purpose of this guideline is to assist nuclear medicine include, but are not limited to, the following: practitioners in recommending, performing, interpreting, and reporting the results of somatostatin receptor scintigra- ● Astrocytomas. phy with 111In-pentetreotide. ● Benign and malignant bone tumors. ● Breast carcinoma. PART II: BACKGROUND INFORMATION AND ● Differentiated thyroid carcinoma (papillary, follicular, DEFINITIONS and Hu¨rthle cell). 111In-pentetreotide is a [111In-DTPA-D-Phe-] conjugate of ● Lymphoma (Hodgkin’s and non-Hodgkin’s). octreotide, a somatostatin analog -

Urocortin 2 Modulates Glucose Utilization and Insulin Sensitivity in Skeletal Muscle
Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle Alon Chen*†, Bhawanjit Brar*, Cheol Soo Choi‡, David Rousso*, Joan Vaughan*, Yael Kuperman†, Shee Ne Kim‡, Cindy Donaldson*, Sean M. Smith*, Pauline Jamieson*, Chien Li*, Tim R. Nagy§, Gerald I. Shulman‡, Kuo-Fen Lee*, and Wylie Vale*¶ *Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, La Jolla, CA 92037; †Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel; ‡Department of Internal Medicine and Cellular and Molecular Physiology and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06536; and §Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL 35294 Contributed by Wylie Vale, August 28, 2006 Skeletal muscle is the principal tissue responsible for insulin- Results and Discussion stimulated glucose disposal and is a major site of peripheral insulin Generation of Ucn 2-Deficient Mice. To explore the physiological resistance. Urocortin 2 (Ucn 2), a member of the corticotropin- role of Ucn 2, we generated mice that are deficient in this releasing factor (CRF) family, and its cognate type 2 CRF receptor peptide. A genomic DNA clone containing Ucn 2 was isolated, (CRFR2) are highly expressed in skeletal muscle. To determine the and a targeting construct in which the full Ucn 2 coding sequence physiological role of Ucn 2, we generated mice that are deficient in was replaced with a neomycin-resistant gene cassette was gen- this peptide. Using glucose-tolerance tests (GTTs), insulin-tolerance erated (Fig. 1a). J1 ES cells were electroporated with the tests (ITTs), and hyperinsulinemic euglycemic glucose clamp stud- targeting construct and were selected as described in ref. -

Insights Into Nuclear G-Protein-Coupled Receptors As Therapeutic Targets in Non-Communicable Diseases
pharmaceuticals Review Insights into Nuclear G-Protein-Coupled Receptors as Therapeutic Targets in Non-Communicable Diseases Salomé Gonçalves-Monteiro 1,2, Rita Ribeiro-Oliveira 1,2, Maria Sofia Vieira-Rocha 1,2, Martin Vojtek 1,2 , Joana B. Sousa 1,2,* and Carmen Diniz 1,2,* 1 Laboratory of Pharmacology, Department of Drug Sciences, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal; [email protected] (S.G.-M.); [email protected] (R.R.-O.); [email protected] (M.S.V.-R.); [email protected] (M.V.) 2 LAQV/REQUIMTE, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal * Correspondence: [email protected] (J.B.S.); [email protected] (C.D.) Abstract: G-protein-coupled receptors (GPCRs) comprise a large protein superfamily divided into six classes, rhodopsin-like (A), secretin receptor family (B), metabotropic glutamate (C), fungal mating pheromone receptors (D), cyclic AMP receptors (E) and frizzled (F). Until recently, GPCRs signaling was thought to emanate exclusively from the plasma membrane as a response to extracellular stimuli but several studies have challenged this view demonstrating that GPCRs can be present in intracellular localizations, including in the nuclei. A renewed interest in GPCR receptors’ superfamily emerged and intensive research occurred over recent decades, particularly regarding class A GPCRs, but some class B and C have also been explored. Nuclear GPCRs proved to be functional and capable of triggering identical and/or distinct signaling pathways associated with their counterparts on the cell surface bringing new insights into the relevance of nuclear GPCRs and highlighting the Citation: Gonçalves-Monteiro, S.; nucleus as an autonomous signaling organelle (triggered by GPCRs). -

Somatostatin Receptor Type 2A Immunohistochemistry in Neuroendocrine Tumors: a Proposal of Scoring System Correlated with Somatostatin Receptor Scintigraphy
Modern Pathology (2007) 20, 1172–1182 & 2007 USCAP, Inc All rights reserved 0893-3952/07 $30.00 www.modernpathology.org Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy Marco Volante1, Maria Pia Brizzi1, Antongiulio Faggiano2, Stefano La Rosa3, Ida Rapa1, Anna Ferrero1, Gelsomina Mansueto4, Luisella Righi1, Silvana Garancini5, Carlo Capella3, Gaetano De Rosa4, Luigi Dogliotti1, Annamaria Colao2 and Mauro Papotti1 1Department of Clinical and Biological Sciences, San Luigi Hospital, University of Turin, Orbassano, Turin, Italy; 2Department of Molecular and Clinical Endocrinology and Oncology, ‘Federico II’ University, Naples, Italy; 3Section of Anatomic Pathology, Department of Human Morphology, University of Insubria and Ospedale di Circolo, Varese, Italy; 4Department of General Pathology, Medicine, Human Pathology and Clinical Pathology, University of Naples ‘Federico II’, Naples, Italy and 5Department of Nuclear Medicine, Ospedale di Circolo, Varese, Italy Typing somatostatin receptor expression in neuroendocrine tumors is of relevance to target somatostatin analogue-based diagnostic approach and treatment. The expanding use of immunohistochemistry to detect somatostatin receptors is to date not paralleled by an accurate methodological setting and standardized interpretation of the results. A multicentric study was designed to compare somatostatin receptor immunohistochemical expression with in vivo scintigraphic data and verify its usefulness in the clinical management of neuroendocrine tumors. After methodological setting by testing different somatostatin receptor antibodies, 107 cases of neuroendocrine tumors with available somatostatin receptor scintigraphy data and pathological material were retrospectively analyzed for somatostatin receptor types 2A, 3 and 5 immunohis- tochemical expression, and compared with scintigraphic images and, whenever available, with the clinical response to somatostatin analogue treatment. -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors
Journal of Nuclear Medicine, published on September 4, 2014 as doi:10.2967/jnumed.114.142000 CONTINUING EDUCATION Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors Clément Morgat1–3, Anil Kumar Mishra2–4, Raunak Varshney4, Michèle Allard1,2,5, Philippe Fernandez1–3, and Elif Hindié1–3 1CHU de Bordeaux, Service de Médecine Nucléaire, Bordeaux, France; 2University of Bordeaux, INCIA, UMR 5287, Talence, France; 3CNRS, INCIA, UMR 5287, Talence, France; 4Division of Cyclotron and Radiopharmaceutical Sciences, Institute of Nuclear Medicine and Allied Sciences, DRDO, New Delhi, India; and 5EPHE, Bordeaux, France Learning Objectives: On successful completion of this activity, participants should be able to list and discuss (1) the presence of bombesin receptors, neurotensin receptors, or neuropeptide-Y receptors in some major tumors; (2) the perspectives offered by radiolabeled peptides targeting these receptors for imaging and therapy; and (3) the choice between agonists and antagonists for tumor targeting and the relevance of various PET radionuclides for molecular imaging. Financial Disclosure: The authors of this article have indicated no relevant relationships that could be perceived as a real or apparent conflict of interest. CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through October 2017. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners.