Membrane Potential
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary - Cellbiology
1 Glossary - Cellbiology Blotting: (Blot Analysis) Widely used biochemical technique for detecting the presence of specific macromolecules (proteins, mRNAs, or DNA sequences) in a mixture. A sample first is separated on an agarose or polyacrylamide gel usually under denaturing conditions; the separated components are transferred (blotting) to a nitrocellulose sheet, which is exposed to a radiolabeled molecule that specifically binds to the macromolecule of interest, and then subjected to autoradiography. Northern B.: mRNAs are detected with a complementary DNA; Southern B.: DNA restriction fragments are detected with complementary nucleotide sequences; Western B.: Proteins are detected by specific antibodies. Cell: The fundamental unit of living organisms. Cells are bounded by a lipid-containing plasma membrane, containing the central nucleus, and the cytoplasm. Cells are generally capable of independent reproduction. More complex cells like Eukaryotes have various compartments (organelles) where special tasks essential for the survival of the cell take place. Cytoplasm: Viscous contents of a cell that are contained within the plasma membrane but, in eukaryotic cells, outside the nucleus. The part of the cytoplasm not contained in any organelle is called the Cytosol. Cytoskeleton: (Gk. ) Three dimensional network of fibrous elements, allowing precisely regulated movements of cell parts, transport organelles, and help to maintain a cell’s shape. • Actin filament: (Microfilaments) Ubiquitous eukaryotic cytoskeletal proteins (one end is attached to the cell-cortex) of two “twisted“ actin monomers; are important in the structural support and movement of cells. Each actin filament (F-actin) consists of two strands of globular subunits (G-Actin) wrapped around each other to form a polarized unit (high ionic cytoplasm lead to the formation of AF, whereas low ion-concentration disassembles AF). -

Biopsychology 2012 – Sec 003 (Dr
Biopsychology 2012 – sec 003 (Dr. Campeau) Study Guide for First Midterm What are some fun facts about the human brain? - there are approximately 100 billion neurons in the brain; - each neuron makes between 1000 to 10000 connections with other neurons; - speed of action potentials varies from less than 1 mph and up to 100 mph. What is a neuron? A very specialized cell type whose function is to receive, process, and send information; these cells are found in the central nervous system (CNS – brain, spinal cord, retina) and the peripheral nervous system (PNS – the rest of the body). What is a nerve? They are axons of individual neurons in bundles or strands of many axons. What are the major parts of a neuron? - cell membrane: “skin” of the neuron; - cytoplasm: everything inside the skin; - nucleus: contains chromosomes (DNA); - ribosomes: generate proteins from mRNA; - mitochondria: energy “generator” of cells (produce ATP); - mitochondria: moves “stuff” inside the neuron (like a tow rope); - soma: cell body, excluding dendrites and axons; - dendrites and spines: part of the neuron usually receiving information from other neurons; - axons: part of the neuron that transmits information to other neurons; - myelin sheath: surrounds axons and provides electrical “insulation”; - nodes of Ranvier: small area on axons devoid of myelin sheath; - presynaptic terminal: area of neuron where neurotransmitter is stored and released by action potentials. What are the different neuron types according to function? 1. Sensory neurons: neurons specialized to “receive” information about the environment. 2. Motor neurons: neurons specialized to produce movement (contraction of muscles). 3. Interneurons or Intrinsic neurons: neurons, usually with short axons, that handle local information. -
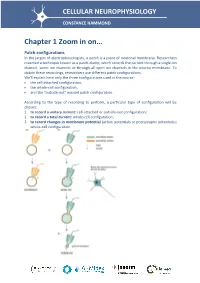
Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 1 Zoom in on… Patch configurations In the jargon of electrophysiologists, a patch is a piece of neuronal membrane. Researchers invented a technique known as a patch-clamp, which records the current through a single ion channel, some ion channels or through all open ion channels in the neuron membrane. To obtain these recordings, researchers use different patch configurations. We'll explain here only the three configurations used in the course: the cell-attached configuration, the whole-cell configuration, and the "outside-out" excised patch configuration. According to the type of recording to perform, a particular type of configuration will be chosen: 1. to record a unitary current: cell-attached or outside-out configuration; 2. to record a total current: whole-cell configuration; 3. to record changes in membrane potential (action potentials or postsynaptic potentials): whole-cell configuration. The cell-attached configuration (attached to the pipette - Figure a) First, the pipette is filled with an extracellular fluid. Positive pressure is applied in the electrode by means of a syringe connected to the electrode, so that the intrapipette fluid tends to leave the pipette. The electrode is then brought near to the soma of a neuron. When the electrode touches the membrane, the positive pressure is withdrawn to draw the membrane toward the mouth of the pipette. We wait without moving the pipette; a seal is made between the walls of the pipette and the soma membrane. This seal strength can be measured by applying a low level of amplitude current in the pipette. Since V = RI, one can measure the resistance of the seal. -

A Thermodynamic Description for Physiological Transmembrane Transport
A thermodynamic description for physiological transmembrane transport Marco Arieli Herrera-Valdez 1 1 Facultad de Ciencias, Universidad Nacional Autónoma de México marcoh@ciencias:unam:mx April 16, 2018 Abstract Physiological mechanisms for passive and active transmembrane transport have been theoretically described using many different approaches. A generic formulation for both passive and active transmem- brane transport, is derived from basic thermodynamical principles taking into account macroscopic forward and backward molecular fluxes, relative to a source compartment, respectively. Electrogenic fluxes also depend on the transmembrane potential and can be readily converted into currents. Interestingly, the conductance-based formulation for current is the linear approximation of the generic formulation for current, around the reversal potential. Also, other known formulas for current based on electrodiffusion turn out to be particular examples of the generic formulation. The applicability of the generic formulations is illustrated with models of transmembrane potential dynamics for cardiocytes and neurons. The generic formulations presented here provide a common ground for the biophysical study of physiological phenomena that depend on transmembrane transport. 1 Introduction One of the most important physiological mechanisms underlying communication within and between cells is the transport of molecules across membranes. Molecules can cross membranes either passively (Stein and Litman, 2014), or via active transport (Bennett, 1956). Passive transmembrane transport occurs through pores that may be spontaneously formed within the lipid bilayer (Blicher and Heimburg, 2013), or within transmembrane proteins called channels (Hille, 1992; Stein and Litman, 2014) that may be selective for particular ion types (Almers and McCleskey, 1984; Doyle et al., 1998; Favre et al., 1996). -

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

Neurotransmitter Actions
Central University of South Bihar Panchanpur, Gaya, India E-Learning Resources Department of Biotechnology NB: These materials are taken/borrowed/modified/compiled from various resources like research articles and freely available internet websites, and are meant to be used solely for the teaching purpose in a public university, and for serving the needs of specified educational programmes. Dr. Jawaid Ahsan Assistant Professor Department of Biotechnology Central University of South Bihar (CUSB) Course Code: MSBTN2003E04 Course Name: Neuroscience Neurotransmitter Actions • Excitatory Action: – A neurotransmitter that puts a neuron closer to an action potential (facilitation) or causes an action potential • Inhibitory Action: – A neurotransmitter that moves a neuron further away from an action potential • Response of neuron: – Responds according to the sum of all the neurotransmitters received at one time Neurotransmitters • Acetylcholine • Monoamines – modified amino acids • Amino acids • Neuropeptides- short chains of amino acids • Depression: – Caused by the imbalances of neurotransmitters • Many drugs imitate neurotransmitters – Ex: Prozac, zoloft, alcohol, drugs, tobacco Release of Neurotransmitters • When an action potential reaches the end of an axon, Ca+ channels in the neuron open • Causes Ca+ to rush in – Cause the synaptic vesicles to fuse with the cell membrane – Release the neurotransmitters into the synaptic cleft • After binding, neurotransmitters will either: – Be destroyed in the synaptic cleft OR – Taken back in to surrounding neurons (reuptake) Excitable cells: Definition: Refers to the ability of some cells to be electrically excited resulting in the generation of action potentials. Neurons, muscle cells (skeletal, cardiac, and smooth), and some endocrine cells (e.g., insulin- releasing pancreatic β cells) are excitable cells. -

Effect of Hyperkalemia on Membrane Potential: Depolarization
❖ CASE 3 A 6-year-old boy is brought to the family physician after his parents noticed that he had difficulty moving his arms and legs after a soccer game. About 10 minutes after leaving the field, the boy became so weak that he could not stand for about 30 minutes. Questioning revealed that he had complained of weakness after eating bananas, had frequent muscle spasms, and occasionally had myotonia, which was expressed as difficulty in releasing his grip or diffi- culty opening his eyes after squinting into the sun. After a thorough physical examination, the boy was diagnosed with hyperkalemic periodic paralysis. The family was advised to feed the boy carbohydrate-rich, low-potassium foods, give him glucose-containing drinks during attacks, and have him avoid strenuous exercise and fasting. ◆ What is the effect of hyperkalemia on cell membrane potential? ◆ What is responsible for the repolarizing phase of an action potential? ◆ What is the effect of prolonged depolarization on the skeletal muscle Na+ channel? 32 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 3: ACTION POTENTIAL Summary: A 6-year-old boy who experiences profound weakness after exer- cise is diagnosed with hyperkalemic periodic paralysis. ◆ Effect of hyperkalemia on membrane potential: Depolarization. ◆ Repolarization mechanisms: Activation of voltage-gated K+ conductance and inactivation of Na+ conductance. ◆ Effect of prolonged depolarization: Inactivation of Na+ channels. CLINICAL CORRELATION Hyperkalemic periodic paralysis (HyperPP) is a dominant inherited trait caused by a mutation in the α subunit of the skeletal muscle Na+ channel. It occurs in approximately 1 in 100,000 people and is more common and more severe in males. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

The Excitable Cell. Resting Membrane Potential and Action Potential (1&2)
The excitable cell. Resting Membrane Potential and Action Potential (1&2) Dr Sergey Kasparov School of Medical Sciences, Room E9 THIS TOPIC IS ALSO COVERED IN TUTORIALS! Teaching home page: http://www.bristol.ac.uk/phys-pharm/media/teaching/ Why do we call these cells “excitable”? Real action potentials.avi The key point: Bioelectricity is generated by the ions moving across cellular membranes Concentration of selected solutes in intracellular fluid and extracellular fluid in millimols mM 160 140 120 100 Insi de the cell 80 60 In extracellular fluid 40 20 0 1 The Na+/K+ pump outside inside [[mMmM]] + + [[mMmM]] + + + + + + + Na 145 + + 15 + K 4 + + 140 ATPATP ••isis an active ion transporter (ATP‐dependent) ••isis responsible for creating and upholding Na+ and K+ concentration gradients ••isis electrogenic – pumps 3 Na+ versus 2 K+ ions Reminder: Movement of ions is affected by concentration gradient and the electrical field Chemical driving force _ _ + + _ + + Electrical driving force _ + + _ + + + _+ _ + _ + + + + _ + _ + _ + + _ Net flux + + + + _ + _ + + V REMINDER: Diffusion through Ion Channels A leak channel A gated channel Both leak and gated channels allow movement of molecules (mainly inorganic ions) down the electrochemical gradient. So, if the gradient reverses, the ions will flow in the opposite direction. 1. The channels are aqueous pores through the membrane. 2. The channels are usually quite selective, for example some only pass Na+, others K+, still others – Cl- 3. Gated channels may be opened or closed by various factors -

9.01 Introduction to Neuroscience Fall 2007
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02) RECITATION #2: Tuesday, September 18th Review of Lectures: 3, 4 Reading: Chapters 3, 4 or Neuroscience: Exploring the Brain (3rd edition) Outline of Recitation: I. Previous Recitation: a. Questions on practice exam questions from last recitation? II. Review of Material: a. Exploiting Axoplasmic Transport b. Types of Glia c. THE RESTING MEMBRANE POTENTIAL d. THE ACTION POTENTIAL III. Practice Exam Questions IV. Questions on Pset? Exploiting Axoplasmic Transport: Maps connections of the brain Rates of transport: - slow: - fast: Examples: Uses anterograde transport: - Uses retrograde transport: - - - Types of Glia: - Microglia: - Astrocytes: - Myelinating Glia: 1 THE RESTING MEMBRANE POTENTIAL: The Cast of Chemicals: The Movement of Ions: Influences by two factors: (1) Diffusion: (2) Electricity: Ohm’s Law: I = gV Ionic Equilibrium Potentials (EION): + Example: ENs * diffusional and electrical forces are equal 2 Nernst Equation: EION = 2.303 RT/zF log [ion]0/[ion]i Calculates equilibrium potential for a SINGLE ion. Inside Outside EION (at 37°C) + [K ] + [Na ] 2+ [Ca ] [Cl ] *Pumps maintain concentration gradients (ex. sodiumpotassium pump; calcium pump) Resting Membrane Potential (VM at rest): Measured resting membrane potential: 65 mV Goldman Equation: + + + + VM = 61.54 mV log (PK[K ]o + PNa[Na ]o)/ (PK[K ]I + PNa[Na ]i) + + Calculates membrane potential when permeable to both Na and K . Remember: at REST, gK >>> gNa therefore, VM is closer to EK THE ACTION POTENTIAL (Nerve Impulse): Phases of an Action Potential: Vm (mV) ENa 0 EK Time 3 Conductance of Ion Channels during AP: Remember: Changes in conductance, or permeability of the membrane to a specific ion, changes the membrane potential. -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -

Neuronal Signaling Neuroscience Fundamentals > the Nerve Cell > the Nerve Cell
Neuronal Signaling Neuroscience Fundamentals > The Nerve Cell > The Nerve Cell NEURONAL SIGNALING SUMMARY ACTION POTENTIALS See: Action Potentials Key Features • All-or-nothing events • Self-propagating • Conduction speed determined by axon diameter (thicker means faster) and amount of myelination (more myelin means faster transmission). CHEMICAL SYNAPSE Steps of Chemical Synapsis 1) The action potential travels down axon of presynaptic neuron. 2) Vesicle fuses with plasma membrane and releases neurotransmitter into the synaptic cleft. 3) Neurotransmitters bind to ligand-gated ion channels, opening them. 4) Ions entering through the open channels cause a depolarization of the membrane, opening voltage-gated ion channels. 5) Ions pass through these voltage-gated ion channels, causing further depolarization of the membrane. 6) This depolarization (action potential) travels along the membrane as more voltage-gated ion channels are opened. ELECTRICAL SYNAPSE • Cytoplasms of adjacent neurons are connected, allowing ions (and action potentials) to travel directly to the next cell. NEURONAL CODING OF STIMULUS STRENGTH Action Potential Frequency • Stimulus strength is based on frequency of action potentials. Action Potential Strength • Strong stimuli can produce another action potential during the relative refractory period. 1 / 7 ABSOLUTE REFRACTORY PERIOD No further action potentials • Voltage-gated sodium channels are open or inactivated so another stimulus, no matter its strength, CANNOT elicit another action potential. RELATIVE REFRACTORY