Regulation of the General Stress Response of Bacillus Subtilis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decomposing Deltahedra
Decomposing Deltahedra Eva Knoll EK Design ([email protected]) Abstract Deltahedra are polyhedra with all equilateral triangular faces of the same size. We consider a class of we will call ‘regular’ deltahedra which possess the icosahedral rotational symmetry group and have either six or five triangles meeting at each vertex. Some, but not all of this class can be generated using operations of subdivision, stellation and truncation on the platonic solids. We develop a method of generating and classifying all deltahedra in this class using the idea of a generating vector on a triangular grid that is made into the net of the deltahedron. We observed and proved a geometric property of the length of these generating vectors and the surface area of the corresponding deltahedra. A consequence of this is that all deltahedra in our class have an integer multiple of 20 faces, starting with the icosahedron which has the minimum of 20 faces. Introduction The Japanese art of paper folding traditionally uses square or sometimes rectangular paper. The geometric styles such as modular Origami [4] reflect that paradigm in that the folds are determined by the geometry of the paper (the diagonals and bisectors of existing angles and lines). Using circular paper creates a completely different design structure. The fact that chords of radial length subdivide the circumference exactly 6 times allows the use of a 60 degree grid system [5]. This makes circular Origami a great tool to experiment with deltahedra (Deltahedra are polyhedra bound by equilateral triangles exclusively [3], [8]). After the barn-raising of an endo-pentakis icosi-dodecahedron (an 80 faced regular deltahedron) [Knoll & Morgan, 1999], an investigation of related deltahedra ensued. -

Convex Polytopes and Tilings with Few Flag Orbits
Convex Polytopes and Tilings with Few Flag Orbits by Nicholas Matteo B.A. in Mathematics, Miami University M.A. in Mathematics, Miami University A dissertation submitted to The Faculty of the College of Science of Northeastern University in partial fulfillment of the requirements for the degree of Doctor of Philosophy April 14, 2015 Dissertation directed by Egon Schulte Professor of Mathematics Abstract of Dissertation The amount of symmetry possessed by a convex polytope, or a tiling by convex polytopes, is reflected by the number of orbits of its flags under the action of the Euclidean isometries preserving the polytope. The convex polytopes with only one flag orbit have been classified since the work of Schläfli in the 19th century. In this dissertation, convex polytopes with up to three flag orbits are classified. Two-orbit convex polytopes exist only in two or three dimensions, and the only ones whose combinatorial automorphism group is also two-orbit are the cuboctahedron, the icosidodecahedron, the rhombic dodecahedron, and the rhombic triacontahedron. Two-orbit face-to-face tilings by convex polytopes exist on E1, E2, and E3; the only ones which are also combinatorially two-orbit are the trihexagonal plane tiling, the rhombille plane tiling, the tetrahedral-octahedral honeycomb, and the rhombic dodecahedral honeycomb. Moreover, any combinatorially two-orbit convex polytope or tiling is isomorphic to one on the above list. Three-orbit convex polytopes exist in two through eight dimensions. There are infinitely many in three dimensions, including prisms over regular polygons, truncated Platonic solids, and their dual bipyramids and Kleetopes. There are infinitely many in four dimensions, comprising the rectified regular 4-polytopes, the p; p-duoprisms, the bitruncated 4-simplex, the bitruncated 24-cell, and their duals. -
![[ENTRY POLYHEDRA] Authors: Oliver Knill: December 2000 Source: Translated Into This Format from Data Given In](https://docslib.b-cdn.net/cover/6670/entry-polyhedra-authors-oliver-knill-december-2000-source-translated-into-this-format-from-data-given-in-1456670.webp)
[ENTRY POLYHEDRA] Authors: Oliver Knill: December 2000 Source: Translated Into This Format from Data Given In
ENTRY POLYHEDRA [ENTRY POLYHEDRA] Authors: Oliver Knill: December 2000 Source: Translated into this format from data given in http://netlib.bell-labs.com/netlib tetrahedron The [tetrahedron] is a polyhedron with 4 vertices and 4 faces. The dual polyhedron is called tetrahedron. cube The [cube] is a polyhedron with 8 vertices and 6 faces. The dual polyhedron is called octahedron. hexahedron The [hexahedron] is a polyhedron with 8 vertices and 6 faces. The dual polyhedron is called octahedron. octahedron The [octahedron] is a polyhedron with 6 vertices and 8 faces. The dual polyhedron is called cube. dodecahedron The [dodecahedron] is a polyhedron with 20 vertices and 12 faces. The dual polyhedron is called icosahedron. icosahedron The [icosahedron] is a polyhedron with 12 vertices and 20 faces. The dual polyhedron is called dodecahedron. small stellated dodecahedron The [small stellated dodecahedron] is a polyhedron with 12 vertices and 12 faces. The dual polyhedron is called great dodecahedron. great dodecahedron The [great dodecahedron] is a polyhedron with 12 vertices and 12 faces. The dual polyhedron is called small stellated dodecahedron. great stellated dodecahedron The [great stellated dodecahedron] is a polyhedron with 20 vertices and 12 faces. The dual polyhedron is called great icosahedron. great icosahedron The [great icosahedron] is a polyhedron with 12 vertices and 20 faces. The dual polyhedron is called great stellated dodecahedron. truncated tetrahedron The [truncated tetrahedron] is a polyhedron with 12 vertices and 8 faces. The dual polyhedron is called triakis tetrahedron. cuboctahedron The [cuboctahedron] is a polyhedron with 12 vertices and 14 faces. The dual polyhedron is called rhombic dodecahedron. -

The Gnomonic Projection
Gnomonic Projections onto Various Polyhedra By Brian Stonelake Table of Contents Abstract ................................................................................................................................................... 3 The Gnomonic Projection .................................................................................................................. 4 The Polyhedra ....................................................................................................................................... 5 The Icosahedron ............................................................................................................................................. 5 The Dodecahedron ......................................................................................................................................... 5 Pentakis Dodecahedron ............................................................................................................................... 5 Modified Pentakis Dodecahedron ............................................................................................................. 6 Equilateral Pentakis Dodecahedron ........................................................................................................ 6 Triakis Icosahedron ....................................................................................................................................... 6 Modified Triakis Icosahedron ................................................................................................................... -

Spectral Realizations of Graphs
SPECTRAL REALIZATIONS OF GRAPHS B. D. S. \DON" MCCONNELL 1. Introduction 2 The boundary of the regular hexagon in R and the vertex-and-edge skeleton of the regular tetrahe- 3 dron in R , as geometric realizations of the combinatorial 6-cycle and complete graph on 4 vertices, exhibit a significant property: Each automorphism of the graph induces a \rigid" isometry of the figure. We call such a figure harmonious.1 Figure 1. A pair of harmonious graph realizations (assuming the latter in 3D). Harmonious realizations can have considerable value as aids to the intuitive understanding of the graph's structure, but such realizations are generally elusive. This note explains and explores a proposition that provides a straightforward way to generate an entire family of harmonious realizations of any graph: A matrix whose rows form an orthogonal basis of an eigenspace of a graph's adjacency matrix has columns that serve as coordinate vectors of the vertices of an harmonious realization of the graph. This is a (projection of a) spectral realization. The hundreds of diagrams in Section 42 illustrate that spectral realizations of graphs with a high degree of symmetry can have great visual appeal. Or, not: they may exist in arbitrarily-high- dimensional spaces, or they may appear as an uninspiring jumble of points in one-dimensional space. In most cases, they collapse vertices and even edges into single points, and are therefore only very rarely faithful. Nevertheless, spectral realizations can often provide useful starting points for visualization efforts. (A basic Mathematica recipe for computing (projected) spectral realizations appears at the end of Section 3.) Not every harmonious realization of a graph is spectral. -

Marvelous Modular Origami
www.ATIBOOK.ir Marvelous Modular Origami www.ATIBOOK.ir Mukerji_book.indd 1 8/13/2010 4:44:46 PM Jasmine Dodecahedron 1 (top) and 3 (bottom). (See pages 50 and 54.) www.ATIBOOK.ir Mukerji_book.indd 2 8/13/2010 4:44:49 PM Marvelous Modular Origami Meenakshi Mukerji A K Peters, Ltd. Natick, Massachusetts www.ATIBOOK.ir Mukerji_book.indd 3 8/13/2010 4:44:49 PM Editorial, Sales, and Customer Service Office A K Peters, Ltd. 5 Commonwealth Road, Suite 2C Natick, MA 01760 www.akpeters.com Copyright © 2007 by A K Peters, Ltd. All rights reserved. No part of the material protected by this copyright notice may be reproduced or utilized in any form, electronic or mechanical, including photo- copying, recording, or by any information storage and retrieval system, without written permission from the copyright owner. Library of Congress Cataloging-in-Publication Data Mukerji, Meenakshi, 1962– Marvelous modular origami / Meenakshi Mukerji. p. cm. Includes bibliographical references. ISBN 978-1-56881-316-5 (alk. paper) 1. Origami. I. Title. TT870.M82 2007 736΄.982--dc22 2006052457 ISBN-10 1-56881-316-3 Cover Photographs Front cover: Poinsettia Floral Ball. Back cover: Poinsettia Floral Ball (top) and Cosmos Ball Variation (bottom). Printed in India 14 13 12 11 10 10 9 8 7 6 5 4 3 2 www.ATIBOOK.ir Mukerji_book.indd 4 8/13/2010 4:44:50 PM To all who inspired me and to my parents www.ATIBOOK.ir Mukerji_book.indd 5 8/13/2010 4:44:50 PM www.ATIBOOK.ir Contents Preface ix Acknowledgments x Photo Credits x Platonic & Archimedean Solids xi Origami Basics xii -
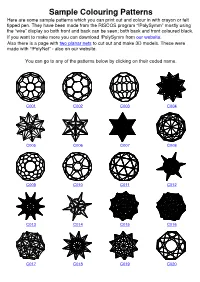
Sample Colouring Patterns Here Are Some Sample Patterns Which You Can Print out and Colour in with Crayon Or Felt Tipped Pen
Sample Colouring Patterns Here are some sample patterns which you can print out and colour in with crayon or felt tipped pen. They have been made from the RISCOS program “!PolySymm” mostly using the “wire” display so both front and back can be seen; both back and front coloured black. If you want to make more you can download !PolySymm from our website. Also there is a page with two planar nets to cut out and make 3D models. These were made with “!PolyNet” - also on our website. You can go to any of the patterns below by clicking on their coded name. C001 C002 C003 C004 C005 C006 C007 C008 C009 C010 C011 C012 C013 C014 C015 C016 C017 C018 C019 C020 Colouring Pattern (C001) Name: truncated icosahedron looking along a 5-fold symmetry Dual: pentakis dodecahedron 60 vertices, 32 faces, 90 edges © Fortran Friends 2016 Colouring Pattern (C002) Name: truncated icosahedron looking along a 3 fold symmetry Dual: pentakis dodecahedron 60 vertices, 32 faces, 90 edges © Fortran Friends 2016 Colouring Pattern (C003) Name: truncated icosahedron looking along a 2 fold symmetry Dual: pentakis dodecahedron 60 vertices, 32 faces, 90 edges © Fortran Friends 2016 Colouring Pattern (C004) Name: great icosahedron looking along 5-fold symmetry Alias: stellation of the icosahedron 92 vertices, 180 faces, 270 edges © Fortran Friends 2016 Colouring Pattern (C005) Name: great icosahedron looking along 5-fold symmetry front only Alias: stellation of the icosahedron 92 vertices, 180 faces, 270 edges © Fortran Friends 2016 Colouring Pattern (C006) Name: great icosahedron -

Chemistry for the 21St Century Edited by F
Chemistry for the 21st Century Edited by f. Keinan, 1. Schechter Chemistry for the 21st Century Edited by Ehud Keincrn, /me/Schechter ~WILEYVCH Weinheim - New-York - Chichester - Brisbane - Singapore - Toronto The Editors ofthis Volume This book was carefully produced. Never- theless, authors and publisher do not Pro$ Dr. E. Keinan warrant the information contained therein Department of Chemistry to be free of errors. Readers are advised Technion - Israel Institute ofTechnology to keep in mind that statements, data, Haifa 32 000 illustrations,procedural details or other Israel items may inadvertently be inaccurate. Library ofcongress Card No.: The Scripps Research Institute applied for 10550 North Torrey Pines Road, MB20 La Jolla BritishLibrary Cataloguingin-Publication Data Ca 92037 A catalogue record for this book is USA availablefromthe British Library. Pro$ Dr. 1. Schechter Die Deutsche Bibliothek- CIP Cataloguing Department of Chemistry in-Publication Data Technion - Israel Institute of Technology A catalogue record for this publication is Haifa 32 000 available from Die Deutsche Bibliothek Israel 0 2001 WILEY-VCH GmbH, Weinheim All rights reserved (includingthose of trans- lation in other languages). No part of this book may be reproduced in any form - by photoprinting,microfilm, or any other means - nor transmitted or translated into a machine language without written permis- sion from the publisher. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law. Printed in the Federal Republic of Germany Printed on acid-free paper Composition IWm & Weyh, Freiburg Printing Strauss Offsetdmck GmbH, Morlenbach Bookbinding Wilh. Osswald 61 Co., Neustadt (Weinstrage) ISBN 3-527-30235-2 I” Contents 1 Some Reflections on Chemistry - Molecular, Supramolecular and Beyond 1 1.1 From Structure to Information. -

Discontinuous Quantum and Classical Magnetic Response of the Pentakis Dodecahedron
Discontinuous quantum and classical magnetic response of the pentakis dodecahedron N. P. Konstantinidis Department of Mathematics and Natural Sciences, The American University of Iraq, Sulaimani, Kirkuk Main Road, Sulaymaniyah, Kurdistan Region, Iraq (Dated: July 6, 2021) The pentakis dodecahedron, the dual of the truncated icosahedron, consists of 60 edge-sharing triangles. It has 20 six-fold and 12 five-fold coordinated vertices, with the former forming a dodec- ahedron, and each of the latter connected to the vertices of one of the 12 pentagons of the dodec- ahedron. When spins mounted on the vertices of the pentakis dodecahedron interact according to the nearest-neighbor antiferromagnetic Heisenberg model, the two different vertex types necessitate the introduction of two exchange constants. As the relative strength of the two constants is varied the molecule interpolates between the dodecahedron and a molecule consisting only of quadran- gles. The competition between the two exchange constants, frustration, and an external magnetic field results in a multitude of ground-state magnetization and susceptibility discontinuities. At the classical level the maximum is ten magnetization and one susceptibility discontinuities when the 12 five-fold vertices interact with the dodecahedron spins with approximately one-half the strength of their interaction. When the two interactions are approximately equal in strength the number of discontinuities is also maximized, with three of the magnetization and eight of the susceptibility. At 1 the full quantum limit, where the magnitude of the spins equals 2 , there can be up to three ground- state magnetization jumps that have the z-component of the total spin changing by ∆Sz = 2, even though quantum fluctuations rarely allow discontinuities of the magnetization. -

Construction of Fractals Based on Catalan Solids Andrzej Katunina*
I.J. Mathematical Sciences and Computing, 2017, 4, 1-7 Published Online November 2017 in MECS (http://www.mecs-press.net) DOI: 10.5815/ijmsc.2017.04.01 Available online at http://www.mecs-press.net/ijmsc Construction of Fractals based on Catalan Solids Andrzej Katunina* aInstitute of Fundamentals of Machinery Design, Silesian University of Technology, 18A Konarskiego Street, 44-100 Gliwice, Poland Received: 17 June 2017; Accepted: 18 September 2017; Published: 08 November 2017 Abstract The deterministic fractals play an important role in computer graphics and mathematical sciences. The understanding of construction of such fractals, especially an ability of fractals construction from various types of polytopes is of crucial importance in several problems related both to the pure mathematical issues as well as some issues of theoretical physics. In the present paper the possibility of construction of fractals based on the Catalan solids is presented and discussed. The method and algorithm of construction of polyhedral strictly deterministic fractals is presented. It is shown that the fractals can be constructed only from a limited number of the Catalan solids due to the specific geometric properties of these solids. The contraction ratios and fractal dimensions are presented for existing fractals with adjacent contractions constructed based on the Catalan solids. Index Terms: Deterministic fractals, iterated function system, Catalan solids. © 2017 Published by MECS Publisher. Selection and/or peer review under responsibility of the Research Association of Modern Education and Computer Science 1. Introduction The fractals, due to their self-similar nature, become playing an important role in many scientific and technical applications. -

Complementary Relationship Between the Golden Ratio and the Silver
Complementary Relationship between the Golden Ratio and the Silver Ratio in the Three-Dimensional Space & “Golden Transformation” and “Silver Transformation” applied to Duals of Semi-regular Convex Polyhedra September 1, 2010 Hiroaki Kimpara 1. Preface The Golden Ratio and the Silver Ratio 1 : serve as the fundamental ratio in the shape of objects and they have so far been considered independent of each other. However, the author has found out, through application of the “Golden Transformation” of rhombic dodecahedron and the “Silver Transformation” of rhombic triacontahedron, that these two ratios are closely related and complementary to each other in the 3-demensional space. Duals of semi-regular polyhedra are classified into 5 patterns. There exist 2 types of polyhedra in each of 5 patterns. In case of the rhombic polyhedra, they are the dodecahedron and the triacontahedron. In case of the trapezoidal polyhedral, they are the icosidodecahedron and the hexecontahedron. In case of the pentagonal polyhedra, they are icositetrahedron and the hexecontahedron. Of these 2 types, the one with the lower number of faces is based on the Silver Ratio and the one with the higher number of faces is based on the Golden Ratio. The “Golden Transformation” practically means to replace each face of: (1) the rhombic dodecahedron with the face of the rhombic triacontahedron, (2) trapezoidal icosidodecahedron with the face of the trapezoidal hexecontahedron, and (3) the pentagonal icositetrahedron with the face of the pentagonal hexecontahedron. These replacing faces are all partitioned into triangles. Similarly, the “Silver Transformation” means to replace each face of: (1) the rhombic triacontahedron with the face of the rhombic dodecahedron, (2) trapezoidal hexecontahedron with the face of the trapezoidal icosidodecahedron, and (3) the pentagonal hexecontahedron with the face of the pentagonal icositetrahedron. -

Fullerenes: Applications and Generalizations Michel Deza Ecole Normale Superieure, Paris Mathieu Dutour Sikiric´ Rudjer Boskovi˘ C´ Institute, Zagreb, and ISM, Tokyo
Fullerenes: applications and generalizations Michel Deza Ecole Normale Superieure, Paris Mathieu Dutour Sikiric´ Rudjer Boskovi˘ c´ Institute, Zagreb, and ISM, Tokyo – p. 1 I. General setting – p. 2 Definition A fullerene Fn is a simple polyhedron (putative carbon molecule) whose n vertices (carbon atoms) are arranged in n 12 pentagons and ( 2 − 10) hexagons. 3 The 2 n edges correspond to carbon-carbon bonds. Fn exist for all even n ≥ 20 except n = 22. 1, 2, 3,..., 1812 isomers Fn for n = 20, 28, 30,. , 60. preferable fullerenes, Cn, satisfy isolated pentagon rule. C60(Ih), C80(Ih) are only icosahedral (i.e., with symmetry Ih or I) fullerenes with n ≤ 80 vertices – p. 3 buckminsterfullerene C60(Ih) F36(D6h) truncated icosahedron, elongated hexagonal barrel soccer ball F24(D6d) – p. 4 45 4590 34 1267 2378 15 12 23 3489 1560 15 34 45 1560 3489 2378 1267 4590 23 12 Dodecahedron Graphite lattice 1 F20(Ih) → 2 H10 F → Z3 the smallest fullerene the “largest”∞ (infinite) Bonjour fullerene – p. 5 Small fullerenes 24, D6d 26, D3h 28, D2 28, Td 30, D5h 30, C2v 30, D2v – p. 6 A C540 – p. 7 What nature wants? Fullerenes Cn or their duals Cn∗ appear in architecture and nanoworld: Biology: virus capsids and clathrine coated vesicles Organic (i.e., carbon) Chemistry also: (energy) minimizers in Thomson problem (for n unit charged particles on sphere) and Skyrme problem (for given baryonic number of nucleons); maximizers, in Tammes problem, of minimum distance between n points on sphere Simple polyhedra with given number of faces, which are the “best” approximation of sphere? Conjecture: FULLERENES – p.