Phylum Nemertea.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Platyhelminthes, Nemertea, and "Aschelminthes" - A
BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. III - Platyhelminthes, Nemertea, and "Aschelminthes" - A. Schmidt-Rhaesa PLATYHELMINTHES, NEMERTEA, AND “ASCHELMINTHES” A. Schmidt-Rhaesa University of Bielefeld, Germany Keywords: Platyhelminthes, Nemertea, Gnathifera, Gnathostomulida, Micrognathozoa, Rotifera, Acanthocephala, Cycliophora, Nemathelminthes, Gastrotricha, Nematoda, Nematomorpha, Priapulida, Kinorhyncha, Loricifera Contents 1. Introduction 2. General Morphology 3. Platyhelminthes, the Flatworms 4. Nemertea (Nemertini), the Ribbon Worms 5. “Aschelminthes” 5.1. Gnathifera 5.1.1. Gnathostomulida 5.1.2. Micrognathozoa (Limnognathia maerski) 5.1.3. Rotifera 5.1.4. Acanthocephala 5.1.5. Cycliophora (Symbion pandora) 5.2. Nemathelminthes 5.2.1. Gastrotricha 5.2.2. Nematoda, the Roundworms 5.2.3. Nematomorpha, the Horsehair Worms 5.2.4. Priapulida 5.2.5. Kinorhyncha 5.2.6. Loricifera Acknowledgements Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS This chapter provides information on several basal bilaterian groups: flatworms, nemerteans, Gnathifera,SAMPLE and Nemathelminthes. CHAPTERS These include species-rich taxa such as Nematoda and Platyhelminthes, and as taxa with few or even only one species, such as Micrognathozoa (Limnognathia maerski) and Cycliophora (Symbion pandora). All Acanthocephala and subgroups of Platyhelminthes and Nematoda, are parasites that often exhibit complex life cycles. Most of the taxa described are marine, but some have also invaded freshwater or the terrestrial environment. “Aschelminthes” are not a natural group, instead, two taxa have been recognized that were earlier summarized under this name. Gnathifera include taxa with a conspicuous jaw apparatus such as Gnathostomulida, Micrognathozoa, and Rotifera. Although they do not possess a jaw apparatus, Acanthocephala also belong to Gnathifera due to their epidermal structure. ©Encyclopedia of Life Support Systems (EOLSS) BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -
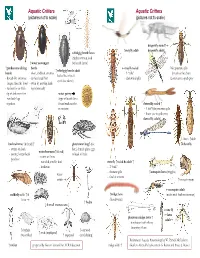
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Comparative Neuroanatomy of Mollusks and Nemerteans in the Context of Deep Metazoan Phylogeny
Comparative Neuroanatomy of Mollusks and Nemerteans in the Context of Deep Metazoan Phylogeny Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades einer Doktorin der Naturwissenschaften genehmigte Dissertation vorgelegt von Diplom-Biologin Simone Faller aus Frankfurt am Main Berichter: Privatdozent Dr. Rudolf Loesel Universitätsprofessor Dr. Peter Bräunig Tag der mündlichen Prüfung: 09. März 2012 Diese Dissertation ist auf den Internetseiten der Hochschulbibliothek online verfügbar. Contents 1 General Introduction 1 Deep Metazoan Phylogeny 1 Neurophylogeny 2 Mollusca 5 Nemertea 6 Aim of the thesis 7 2 Neuroanatomy of Minor Mollusca 9 Introduction 9 Material and Methods 10 Results 12 Caudofoveata 12 Scutopus ventrolineatus 12 Falcidens crossotus 16 Solenogastres 16 Dorymenia sarsii 16 Polyplacophora 20 Lepidochitona cinerea 20 Acanthochitona crinita 20 Scaphopoda 22 Antalis entalis 22 Entalina quinquangularis 24 Discussion 25 Structure of the brain and nerve cords 25 Caudofoveata 25 Solenogastres 26 Polyplacophora 27 Scaphopoda 27 i CONTENTS Evolutionary considerations 28 Relationship among non-conchiferan molluscan taxa 28 Position of the Scaphopoda within Conchifera 29 Position of Mollusca within Protostomia 30 3 Neuroanatomy of Nemertea 33 Introduction 33 Material and Methods 34 Results 35 Brain 35 Cerebral organ 38 Nerve cords and peripheral nervous system 38 Discussion 38 Peripheral nervous system 40 Central nervous system 40 In search for the urbilaterian brain 42 4 General Discussion 45 Evolution of higher brain centers 46 Neuroanatomical glossary and data matrix – Essential steps toward a cladistic analysis of neuroanatomical data 49 5 Summary 53 6 Zusammenfassung 57 7 References 61 Danksagung 75 Lebenslauf 79 ii iii 1 General Introduction Deep Metazoan Phylogeny The concept of phylogeny follows directly from the theory of evolution as published by Charles Darwin in The origin of species (1859). -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

A Phylum-Wide Survey Reveals Multiple Independent Gains of Head Regeneration Ability in Nemertea
bioRxiv preprint doi: https://doi.org/10.1101/439497; this version posted October 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. A phylum-wide survey reveals multiple independent gains of head regeneration ability in Nemertea Eduardo E. Zattara1,2,5, Fernando A. Fernández-Álvarez3, Terra C. Hiebert4, Alexandra E. Bely2 and Jon L. Norenburg1 1 Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA 2 Department of Biology, University of Maryland, College Park, MD, USA 3 Institut de Ciències del Mar, Consejo Superior de Investigaciones Científicas, Barcelona, Spain 4 Institute of Ecology and Evolution, University of Oregon, Eugene, OR, USA 5 INIBIOMA, Consejo Nacional de Investigaciones Científicas y Tecnológicas, Bariloche, RN, Argentina Corresponding author: E.E. Zattara, [email protected] Abstract Animals vary widely in their ability to regenerate, suggesting that regenerative abilities have a rich evolutionary history. However, our understanding of this history remains limited because regeneration ability has only been evaluated in a tiny fraction of species. Available comparative regeneration studies have identified losses of regenerative ability, yet clear documentation of gains is lacking. We surveyed regenerative ability in 34 species spanning the phylum Nemertea, assessing the ability to regenerate heads and tails either through our own experiments or from literature reports. Our sampling included representatives of the 10 most diverse families and all three orders comprising this phylum. -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

Invertebrates Invertebrates: • Are Animals Without Backbones • Represent 95% of the Animal Kingdom Animal Diversity Morphological Vs
Invertebrates Invertebrates: • Are animals without backbones • Represent 95% of the animal kingdom Animal Diversity Morphological vs. Molecular Character Phylogeny? A tree is a hypothesis supported or not supported by evidence. Groupings change as new evidence become available. Sponges - Porifera Natural Bath Sponges – over-collected, now uncommon Sponges • Perhaps oldest animal phylum (Ctenphora possibly older) • may represent several old phyla, some now extinct ----------------Ctenophora? Sponges - Porifera • Mostly marine • Sessile animals • Lack true tissues; • Have only a few cell types, cells kind of independent • Most have no symmetry • Body resembles a sac perforated with holes, system of canals. • Strengthened by fibers of spongin, spicules Sponges have a variety of shapes Sponges Pores Choanocyte Amoebocyte (feeding cell) Skeletal Water fiber flow Central cavity Flagella Choanocyte in contact with an amoebocyte Sponges - Porifera • Sessile filter feeder • No mouth • Sac-like body, perforated by pores. • Interior lined by flagellated cells (choanocytes). Flagellated collar cells generate a current, draw water through the walls of the sponge where food is collected. • Amoeboid cells move around in the mesophyll and distribute food. Sponges - Porifera Grantia x.s. Sponge Reproduction Asexual reproduction • Fragmentation or by budding. • Sponges are capable of regeneration, growth of a whole from a small part. Sexual reproduction • Hermaphrodites, produce both eggs and sperm • Eggs and sperm released into the central cavity • Produces -

Evolution of Direct-Developing Larvae: Selection Vs Loss Margaret Snoke Smith,1 Kirk S
Hypotheses Evolution of direct-developing larvae: selection vs loss Margaret Snoke Smith,1 Kirk S. Zigler,2 and Rudolf A. Raff1,3* Summary echinoderms, sea urchins, reveal that a majority of sea urchin Observations of a sea urchin larvae show that most species exhibit one of two life history strategies (Fig. 1).(10) species adopt one of two life history strategies. One strategy is to make numerous small eggs, which develop One strategy is to make many small eggs that develop into a into a larva with a required feeding period in the water larva that must feed and grow for weeks or months in the water column before metamorphosis. In contrast, the second column before metamorphosis. In the water column, these strategy is to make fewer large eggs with a larva that does larvae are dependent on plankton abundance for food and are not feed, which reduces the time to metamorphosis and subject to high levels of predation.(11) The other strategy thus the time spent in the water column. The larvae associated with each strategy have distinct morpholo- involves increasing egg size and maternal provisioning, which gies and developmental processes that reflect their decreases the reliance on planktonic food sources and the feeding requirements, so that those that feed exhibit time to metamorphosis, thus minimizing larval mortality. indirect development with a complex larva, and those that However, assuming finite resources for egg production, do not feed form a morphologically simplified larva and increasing egg size also reduces the total number of eggs exhibit direct development. Phylogenetic studies show that, in sea urchins, a feeding larva, the pluteus, is the produced. -

Hookworm-Related Cutaneous Larva Migrans
326 Hookworm-Related Cutaneous Larva Migrans Patrick Hochedez , MD , and Eric Caumes , MD Département des Maladies Infectieuses et Tropicales, Hôpital Pitié-Salpêtrière, Paris, France DOI: 10.1111/j.1708-8305.2007.00148.x Downloaded from https://academic.oup.com/jtm/article/14/5/326/1808671 by guest on 27 September 2021 utaneous larva migrans (CLM) is the most fre- Risk factors for developing HrCLM have specifi - Cquent travel-associated skin disease of tropical cally been investigated in one outbreak in Canadian origin. 1,2 This dermatosis fi rst described as CLM by tourists: less frequent use of protective footwear Lee in 1874 was later attributed to the subcutane- while walking on the beach was signifi cantly associ- ous migration of Ancylostoma larvae by White and ated with a higher risk of developing the disease, Dove in 1929. 3,4 Since then, this skin disease has also with a risk ratio of 4. Moreover, affected patients been called creeping eruption, creeping verminous were somewhat younger than unaffected travelers dermatitis, sand worm eruption, or plumber ’ s itch, (36.9 vs 41.2 yr, p = 0.014). There was no correla- which adds to the confusion. It has been suggested tion between the reported amount of time spent on to name this disease hookworm-related cutaneous the beach and the risk of developing CLM. Consid- larva migrans (HrCLM).5 ering animals in the neighborhood, 90% of the Although frequent, this tropical dermatosis is travelers in that study reported seeing cats on the not suffi ciently well known by Western physicians, beach and around the hotel area, and only 1.5% and this can delay diagnosis and effective treatment. -

A Functional Approach to Resolving the Biogeocomplexity of Two Extreme Environments Haydn Rubelmann III University of South Florida, [email protected]
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 11-12-2014 A Functional Approach to Resolving the Biogeocomplexity of Two Extreme Environments Haydn Rubelmann III University of South Florida, [email protected] Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the Marine Biology Commons, and the Microbiology Commons Scholar Commons Citation Rubelmann, Haydn III, "A Functional Approach to Resolving the Biogeocomplexity of Two Extreme Environments" (2014). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/5432 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. A Functional Approach to Resolving the Biogeocomplexity of Two Extreme Environments by Haydn Rubelmann III A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Cell Biology, Microbiology and Molecular Biology College of Arts and Sciences University of South Florida Major Professor: James R. Garey, Ph.D. Randy Larsen, Ph.D. Kathleen Scott, Ph.D. David Merkler, Ph.D. Date of Approval: November 12, 2014 Keywords: environmental microbiology, extremophiles, shallow-water hydrothermal vents, anoxic marine pits Copyright © 2014, Haydn Rubelmann III DEDICATION I would like to dedicate this dissertation to three of my personal champions: my grandfather, Haydn Rubelmann Sr. (1929 - 2004), who encouraged me to pursue an academic career; my stepfather, Dale Jones (1954 - 2008), who was the best father anyone could ever hope for, and my husband, Eduardo Godoy, who suffered through not only 8 years of my doctoral tenure, but a grueling civil liberty injustice that almost wedged the Caribbean Sea between us. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber .........................................................................