Blood Oxygenation Level-Dependent Magnetic Resonance Imaging
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution of Phthalate Esters in Topsoil: a Case Study in the Yellow River Delta, China
Environ Monit Assess (2013) 185:8489–8500 DOI 10.1007/s10661-013-3190-7 Distribution of phthalate esters in topsoil: a case study in the Yellow River Delta, China Yang Hongjun & Xie Wenjun & Liu Qing & Liu Jingtao & Yu Hongwen & Lu Zhaohua Received: 21 August 2012 /Accepted: 4 April 2013 /Published online: 24 April 2013 # The Author(s) 2013. This article is published with open access at Springerlink.com Abstract TheYellowRiverDelta(YRD)isatypical anthropogenic activities areas (such as the urbanization agricultural and petrochemical industrial area of China. and industrialization), and agriculture influence district, To assess the current status of phthalate esters (PAEs) of which mainly originated from construction waste, mu- soil residues, soil samples (0∼20 cm) (n=82) were col- nicipal sewage, agricultural waste and pesticide, lected in Bincheng District, at the geographic center of discarded plastic effusion and atmospheric depositions. the YRD. PAEs were detected in all topsoil samples Concentrations of PAEs were weakly positivity correlat- analyzed, which indicated that PAEs are ubiquitous en- ed with soil organic carbon content and pH, which vironmental contaminants. Concentrations of 11 PAEs suggested both of them can affect the distribution of are in the range of 0.794∼19.504 μgg−1,withanaverage PAEs. The concentration of di (2-ethylhexyl) phthalate value of 2.975 μgg−1. It was presented that PAEs pollu- and di-n-butyl phthalate dominated in the 11 PAEs, with tion in this area was weak and monotonously increasing the average values of 0.735 and 1.915 μgg−1, respec- along the rural–urban gradient. Higher concentrations tively, and accounted for 92.1 % of the whole PAEs’ were observed from roadsides (and/or gutters), densely concentrations. -

Spatial Optimization and Mode Analysis of Primary Industry Structure in Yellow River Delta Ping Yang, Yujian Yang
Spatial Optimization and Mode Analysis of Primary Industry Structure in Yellow River Delta Ping Yang, Yujian Yang To cite this version: Ping Yang, Yujian Yang. Spatial Optimization and Mode Analysis of Primary Industry Structure in Yellow River Delta. 6th Computer and Computing Technologies in Agriculture (CCTA), Oct 2012, Zhangjiajie, China. pp.150-160, 10.1007/978-3-642-36137-1_19. hal-01348227 HAL Id: hal-01348227 https://hal.inria.fr/hal-01348227 Submitted on 22 Jul 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Spatial Optimization and Mode Analysis of Primary Industry Structure in Yellow River Delta Ping Yang, Yujian Yang* 1 The institute of sustainable development of Shandong Agricultural Science Academy 2S & T Information Engineering Technology Center of Shandong Academy of Agricultural Science, Information center of agronomy College of Shandong University *Corresponding author, Address: S&T Information Engineering Research Center, Number 202 Gongye North Road, -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -
Study on Spatial Model and Service Radius of Rural Areas And
Study on Spatial Model and Service Radius of Rural Areas and Agriculture Information Level in Yellow-river Delta Yujian Yang1, Guangming Liu2, Xueqin Tong1, Zhicheng Wang1 1.S & T Information Engineering Technology Center of Shandong Academy of Agricultural Science, Information center of agronomy College of Shandong University Jinan 250100, P. R. China 2. Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, P. R. China 1 Corresponding author, Address: S&T Information Engineering Research Center, Number 202 Gongye North Road, Licheng District of Jinan, 250100, Shandong Province, P. R. China, Tel: +86-531-83179076, Fax:+86-531-83179821, Email:[email protected] Abstract. Based on the evaluation methods and systems of information measurement level, and according to the principles of agriculture information subject, the study constructed 13 indices system for the measurement of the rural areas and agriculture information level in Yellow-river Delta in 2007. Spatial autocorrelation model of rural areas and agriculture information of 19 country units showed that the comprehensive information level of Hanting, Shouguang, Guangrao, Bincheng, Huimin, Wudi and Yangxin country unit is very significant, has the obvious spatial agglomeration and homogeneity characteristics, but information level agglomeration of Kenli country and Zouping city has the significant heterogeneity, and information level agglomeration characteristics of other 10 country units is not significant. The radius surface of the complicated information level from radial basis function model indicated that rural areas and agriculture information service has a certain service radius, the distance of service radius in theory is 30Km, the gradient and hierarchy is obvious. According to it, the comprehensive service node should be established in Bincheng district and the secondary service node should be set up in Wudi country for improving the service efficiency. -
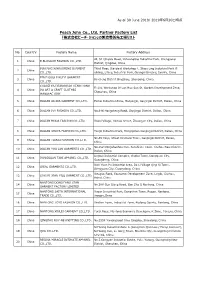
Peach John Co., Ltd. Partner Factory List 「株式会社ピーチ・ジョン」の製造委託先工場リスト
As of 30 June 2019/ 2019年6月30日時点 Peach John Co., Ltd. Partner Factory List 「株式会社ピーチ・ジョン」の製造委託先工場リスト No. Country Factory Name Factory Address 4F, 51 Qingxia Road, Yuhuangling Industrial Park, Chengyang 1 China B.BLOSSOM FASHION CO.,LTD. District, Qingdao, China BINYANG XINBAOFENG GARMENT Third Floor, Standard Workshop 4, Shigu Ling Industrial Park B 2 China CO.,LTD. uilding, Litang Industrial Park, Guangxi Binyang County, China BINZHOUSHI RUIYI GARMENT 3 China Bincheng District Bingzhou, Shandong, China CO.,LTD. CHAOZHOU XIANGQIAO HENG HONG Fl 1/4, Workshop Of Lan Hua San St, Garden Development Zone, 4 China DA ART & CRAFT CLOTHES Chaozhou, China MANUFACTORY 5 China DALIAN AILIDA GARMENT CO.,LTD. Beihai Industrial Zone, Maoyingzi, Ganjingzi District, Dalian, China 6 China DALIAN IVY FASHION CO.,LTD. No.646 Hongcheng Road, Ganjingzi District, Dalian, China 7 China DALIAN MODA FASHION CO.,LTD. Xisosi Village, Xinhua Street, Zhuangue City, Dalian, China 8 China DALIAN SHUYA FASHION CO.,LTD. Tangli Industrial Park, Hongqizhen Ganjingzi District, Dalian, China No.65 Xinyi, Street Xinzhaizi Town, Ganjingzi District, Dalian, 9 China DALIAN TIANAI FASHION CO.,LTD. China No.552 Dongshanhou Cun, Sanshilipu Town, Jinzhou New District, 10 China DALIAN YOU LAN GARMENTS CO.,LTD. Dalian, China Lingwu Industrial Complex, Gaobu Town, Dongguan City, 11 China DONGGUAN TANI APPAREL CO.,LTD. Guangdong, China Xian Yuan Pu Industrial Area, Da Li Village Qing Xi Town, 12 China IDEAL GARMENTS CO.,LTD. Dongguan City, Guangdong, China Xiaoguo Road, Economic Development Zone, Lingbi, Suzhou, 13 China LING BI XIAN YILU GARMENT CO.,LTD. Anhui, China NANTONG DONG FANG STAR 14 China No.388 Guo Qiang Road, Gan Zha Q Nantong, China GARMENT FACTORY LIMITED NANTONG SAITA INTERNATIONAL Xuean Industrial Park, Dongchen Town, Rugao, Nantong, 15 China TRADE CO.,LTD. -

Minimum Wage Standards in China August 11, 2020
Minimum Wage Standards in China August 11, 2020 Contents Heilongjiang ................................................................................................................................................. 3 Jilin ............................................................................................................................................................... 3 Liaoning ........................................................................................................................................................ 4 Inner Mongolia Autonomous Region ........................................................................................................... 7 Beijing......................................................................................................................................................... 10 Hebei ........................................................................................................................................................... 11 Henan .......................................................................................................................................................... 13 Shandong .................................................................................................................................................... 14 Shanxi ......................................................................................................................................................... 16 Shaanxi ...................................................................................................................................................... -

Annual Report
2019 ANNUAL REPORT (A joint stock company incorporated in the People’s Republic of China with limited liability) (H Shares Stock Code: 3866) (Preference Shares Stock Code: 4611) 2019 Contents Section I Important Notice, Contents and Definitions 3 Section II Corporate Information and Key Financial Highlights 6 Section III Chairman’s Statement 13 Section IV President’s Statement 14 Section V Business Overview 15 Section VI Discussion and Analysis of Operations 18 Section VII Significant Events 78 Section VIII Changes in Share Capital and Information on Shareholders 95 Section IX Preference Shares 105 Section X Directors, Supervisors, Senior Management and Employees 108 Section XI Corporate Governance 126 Section XII Report of the Board of Directors 150 Section XIII Report of the Board of Supervisors 157 Section XIV Independent Auditor’s Report 158 Section XV Financial Statements and Notes 165 Section XVI Unaudited Supplementary Financial Information 282 Bank of Qingdao Co., Ltd. 2 2019 Annual Report Section I Important Notice, Contents and Definitions 1. The Board of Directors, Board of Supervisors, Directors, Supervisors and senior management of the Bank assure that the information in this annual report contains no false records, misleading statements or material omissions, and severally and jointly take full responsibility for the authenticity, accuracy and completeness of the information in this annual report. 2. The proposals on the 2019 Annual Report of Bank of Qingdao Co., Ltd. and its summary were considered and approved at the 33rd meeting of the seventh session of the Board of Directors of the Bank held on 20 March 2020. There were 14 Directors eligible for attending the meeting, of whom 14 Directors attended the meeting. -

CHINA the Church of Almighty God: Prisoners Database (1663 Cases)
CHINA The Church of Almighty God: Prisoners Database (1663 cases) Prison term: 15 years HE Zhexun Date of birth: On 18th September 1963 Date and place of arrest: On 10th March 2009, in Xuchang City, Henan Province Charges: Disturbing social order and using a Xie Jiao organization to undermine law enforcement because of being an upper-level leader of The Church of Almighty God in mainland China, who was responsible for the overall work of the church Statement of the defendant: He disagreed with the decision and said what he believed in is not a Xie Jiao. Court decision: In February 2010, he was sentenced to 15 years in prison by the Zhongyuan District People’s Court of Zhengzhou City, Henan Province. Place of imprisonment: No. 1 Prison of Henan Province Other information: He was regarded by the Chinese authorities as a major criminal of the state and had long been on the wanted list. To arrest him, authorities offered 500,000 RMB as a reward to informers who gave tips leading to his arrest to police. He was arrested at the home of a Christian in Xuchang City, Henan Province. Based on the information from a Christian serving his sentence in the same prison, HE Zhexun was imprisoned in a separate area and not allowed to contact other prisoners. XIE Gao, ZOU Yuxiong, SONG Xinling and GAO Qinlin were arrested in succession alongside him and sentenced to prison terms ranging from 11 to 12 years. Source: https://goo.gl/aGkHBj Prison term: 14 years MENG Xiumei Age: Forty-one years old Date and place of arrest: On 14th August 2014, in Xinjiang Uyghur Autonomous Region Charges: Using a Xie Jiao organization to undermine law enforcement because of being a leader of The Church of Almighty God and organizing gatherings for Christians and the work of preaching the gospel in Ili prefecture Statement of the defendant: She claimed that her act did not constitute crimes. -

The Warehouse Ethical Sourcing Report 2021
ETHICAL SOURCING REPORT 2021 2 CONTENTS INTRODUCTION APPENDICES 04. CEO introduction 22. 2019 – 2020 KPI table 05. Programme at a glance 23. Factory policy poster 06. Top 20 source countries 24. Apparel tier 1 factory list 2020 SUMMARY 26. Apparel tier 2 factory list 08. 2020 summary 36. Apparel brand list 10. Her Project updates 37. Other categories factory list Getting the most from this report 12. COVID-19 updates 49. Country wage & working hour table A brief overview of our programme and progress in 2020 can be viewed on pages 5 and 8. Our key areas of focus 13. Responding to forced labour risks and achievements can be found on pages 10 to 20. SUSTAINABLE MATERIALS Finally, the appendices from page 22 reveal performance trends over the past two years, and feature our factory 16. Better Cotton Initiative policy poster, brand and factory lists and source country wage and working hour data. 17. Forest Stewardship Council OUR POLICY IN PRACTICE 19. Policy in practice ETHICAL SOURCING REPORT 2021 INTRODUCTION. ETHICAL SOURCING REPORT 2021 4 CEO’S INTRODUCTION. APPENDICES programme. I believe, given our scale and challenges and achievements to date, diversity, this is the leading programme as well the road ahead. We invite both of its kind within the New Zealand retail your encouragement and suggestions for Ethical Sourcing is just one programme sector. improvement. within The Warehouse’s suite of “Sustainable & affordable” initiatives. Within The Warehouse Group’s 2020 Finally, I want to express my sincere Sustainable & affordable is The POLICY IN PRACTICE POLICY Annual Report, I pledged that COVID-19 gratitude to our ethical sourcing Warehouse’s guiding statement and will not slow down our commitment specialists and their colleagues within branding device representing our to becoming one of New Zealand’s our 200 person strong sourcing team. -

2020 Annual Report Contents
(A joint stock company incorporated in the People’s Republic of China with limited liability) (H Shares Stock Code: 3866) (Preference Shares Stock Code: 4611) 2020 ANNUAL REPORT Contents Section I Important Notice, Contents and Definitions 2 Section II Corporate Information and Key Financial Highlights 5 Section III Chairman’s Statement 12 Section IV President’s Statement 14 Section V Business Overview 17 Section VI Operation Discussion and Analysis 21 Section VII Significant Events 86 Section VIII Changes in Shareholdings and Information on Shareholders 102 Section IX Preference Shares 114 Section X Directors, Supervisors, Senior Management and Employees 116 Section XI Corporate Governance 129 Section XII Report of the Board of Directors 153 Section XIII Report of the Board of Supervisors 160 Section XIV Independent Auditor’s Report 161 Section XV Financial Statements and Notes 168 Section XVI Unaudited Supplementary Financial Information 286 Bank of Qingdao Co., Ltd. 2020 Annual Report 1 Section I Important Notice, Contents and Definitions 1. The Board of Directors, Board of Supervisors, Directors, Supervisors and senior management of the Bank assure that the information in this annual report contains no false records, misleading statements or material omissions, and severally and jointly take full responsibility for the authenticity, accuracy and completeness of the information in this annual report. 2. The proposals on the 2020 Annual Report of Bank of Qingdao Co., Ltd., its summary and the results announcement were considered and approved at the 44th meeting of the seventh session of the Board of Directors of the Bank held on 30 March 2021. There were 14 Directors eligible for attending the meeting, and 14 Directors actually attended the meeting. -

SMR: PRC: Shandong Hai River Basin Pollution Control Project
Social Monitoring Report Project Number: 36505 December 2007 PRC: Shandong Hai River Basin Pollution Control Project Prepared by Shandong Construction Development Research Institute Shandong Province, PRC For Shandong Provincial Project Management Office This report has been submitted to ADB by the Shandong Provincial Government Project Management Office and is made publicly available in accordance with ADB’s public communications policy (2005). It does not necessarily reflect the views of ADB. Asian Bank Loan Project Shandong Haihe Basin Pollution Control Project Monitoring and Evaluation Report of Immigrant Resettlement (No. 2) December 15, 2007 Immigrant Resettlement External Monitoring Group, Shandong Construction Development Research Institute Contents Foreword …………………………………………………………………………1 I. Monitoring and Evaluation Overview………………………………………….2 1.1. Purpose of monitoring and evaluation ………………………………………2 1.2. Contents and methods of monitoring and evaluation ………………………..3 1.3. Organization and implementation of monitoring and evaluation ……………4 II. Monitoring and evaluation of land requisition progress ………………………5 2.1. Monitoring of land requisition progress ………………………………….….5 2.2. Evaluation of land requisition progress ………………………………….…..5 III. Monitoring and Evaluation of Immigration Resettlement and Compensation Progress ………………………………………………………………….………12 IV. Monitoring and evaluation of fund settlement and use………………………12 4.1. Monitoring of fund settlement and use………………………………...……12 4.2. Evaluation of fund settlement and use……………………………...……….15 V. Monitoring and evaluation of production and living conditions of immigrant villages ………………………………………………………………………..…15 5.1. Typical households in immigrant monitoring…………………..…………...15 5.2. Monitoring of production and resettlement of immigrants and affect villagers………………………………………………………………...…......…15 5.3. Monitoring of immigrant living resettlement ……………………….....…..24 5.4. Evaluation of production, living resettlement and income level of affected villagers ………………………………………………………………..……25 VI. -

Vertical Facility List
Facility List The Walt Disney Company is committed to fostering safe, inclusive and respectful workplaces wherever Disney-branded products are manufactured. Numerous measures in support of this commitment are in place, including increased transparency. To that end, we have published this list of the roughly 7,600 facilities in over 70 countries that manufacture Disney-branded products sold, distributed or used in our own retail businesses such as The Disney Stores and Theme Parks, as well as those used in our internal operations. Our goal in releasing this information is to foster collaboration with industry peers, governments, non- governmental organizations and others interested in improving working conditions. Under our International Labor Standards (ILS) Program, facilities that manufacture products or components incorporating Disney intellectual properties must be declared to Disney and receive prior authorization to manufacture. The list below includes the names and addresses of facilities disclosed to us by vendors under the requirements of Disney’s ILS Program for our vertical business, which includes our own retail businesses and internal operations. The list does not include the facilities used only by licensees of The Walt Disney Company or its affiliates that source, manufacture and sell consumer products by and through independent entities. Disney’s vertical business comprises a wide range of product categories including apparel, toys, electronics, food, home goods, personal care, books and others. As a result, the number of facilities involved in the production of Disney-branded products may be larger than for companies that operate in only one or a limited number of product categories. In addition, because we require vendors to disclose any facility where Disney intellectual property is present as part of the manufacturing process, the list includes facilities that may extend beyond finished goods manufacturers or final assembly locations.