Study of Optical Metallurgical Microscope Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Practical Tips for Two-Photon Microscopy
Appendix 1 Practical Tips for Two-Photon Microscopy Mark B. Cannell, Angus McMorland, and Christian Soeller INTRODUCTION blue and green diode lasers. To provide an alignment beam to which the external laser can be aligned, light from this reference As is clear from a number of the chapters in this volume, 2-photon laser needs to be bounced back through the microscope optical microscopy offers many advantages, especially for living-cell train and out through the external coupling port: studies of thick specimens such as brain slices and embryos. CAUTION: Before you switch on the reference laser in this However, these advantages must be balanced against the fact that configuration make sure that all PMTs are protected and/or commercial multiphoton instrumentation is much more costly than turned off. the equipment used for confocal or widefield/deconvolution. Given Place a front-surface mirror on the stage of the microscope and these two facts, it is not surprising that, to an extent much greater focus onto the reflective surface using an air objective for conve- than is true of confocal, many researchers have decided to add a nience (at sharp focus, you should be able to see scratches or other femtosecond (fs) pulsed near-IR laser to a scanner and a micro- mirror defects through the eyepieces). The idea of this method is scope to make their own system (Soeller and Cannell, 1996; Tsai to cause the reference laser beam to bounce back through the et al., 2002; Potter, 2005). Even those who purchase a commercial optical train and emerge from the other laser port. -

Arrangement of a 4Pi Microscope for Reducing the Confocal Detection
Arrangement of a 4Pi microscope for reducing the confocal detection volume with two-photon excitation Nicolas Sandeau∗and Hugues Giovannini† Received 16 November 2005; received in revised form 7 February 2006; accepted 8 February 2006 Institut Fresnel, UMR 6133 CNRS, Universit´ePaul C´ezanne Aix-Marseille III, F-13397 Marseille cedex 20, France The main advantage of two-photon fluorescence confocal microscopy is the low absorption obtained with live tissues at the wavelengths of operation. However, the resolution of two-photon fluorescence confocal microscopes is lower than in the case of one-photon excitation. The 4Pi microscope type C working in two-photon regime, in which the excitation beams are coherently superimposed and, simultaneously, the emitted beams are also coherently added, has shown to be a good solution for increasing the resolution along the optical axis and for reducing the amplitude of the side lobes of the point spread function. However, the resolution in the transverse plane is poorer than in the case of one-photon excitation due to the larger wavelength in- volved in the two-photon fluorescence process. In this paper we show that a particular arrangement of the 4Pi microscope, referenced as 4Pi’ microscope, is a solution for obtaining a lateral resolution in the two-photon regime sim- ilar or even better to that obtained with 4Pi microscopes working in the arXiv:physics/0610036v1 [physics.bio-ph] 6 Oct 2006 one-photon excitation regime. Keywords: Resolution; Fluorescence microscopy; 4Pi microscopy; Confocal; Detection volume; Two-photon excitation ∗E-mail: [email protected] †E-mail: [email protected]; Tel: +33 491 28 80 66; Fax: +33 491 28 80 67 1 1 Introduction Strong efforts have been made in the last decade to improve the resolution of fluorescence microscopes. -

NNT '09 Program
NNT '09 Program 9-Nov Wednesday, November 11 12:00 Exhibit set-up, Room J2/J3, San Jose Convention Center 12:00 Registration 17:00 Welcome Reception and Equipment Exhibit, Room J2/J3, San Jose Convention Center Thursday, November 12, Room J1/J4 , San Jose Convention Center Plenary Session - Session Chairs: S. Chou (Princeton) & L. Montelius (Lund) 8:15 Welcome: Stephen Chou and Christie Marrian 8:30 Plenary NNT is Losing the Propaganda War Fabian Pease Stanford University 9:00 Invited 1 Template Infrastructure for Nanoimprint Lithography Nobuhito Toyama Dai Nippon Printing 9:20 Invited 2 NaPANIL: Consolidation of Nanoimprinting for Production Jouni Ahopelto VTT Microsystems and Nanoelectronics 9:40 Invited 3 Shrink-Induced Nanostructures Michelle Khine University of California, Irvine 10:00 Break Magnetics/Biology/Solar - Session Chairs: G. Willson (U. Texas) & H. Schift (PSI) 10:30 Invited 4 The Nano-imprinting Process towards Patterned Media Manufacturing Tsai-Wei Wu HGST 10:50 c1 Large Scale Fabrication of Nanoimprinted Magnetic Nanoparticles with Self-Assembled Templates Wei Hu Stanford University 11:05 c2 Photocatalytic Nanolithography: An Emergent Patterning Technique Relevant to Biotechnology Jane P Bearinger LLNL 11:20 c3 Fabrication of 3D Cell Containers with Integrated Topography by Combined Microscale Arne Schleunitz Paul Scherrer Institut Thermoforming and Thermal Nanoimprint 11:35 c4 Nanoimprinting of Subphthalocyanines for Photovoltaic Applications Xiaogan Liang Lawrence Berkeley National Laboratory 11:50 c5 Nanopatterned anode for organic solar cell by nanoimprint Dae-Geun Choi Korea Institute of Machinery & Materials 12:05 Lunch Electronics/Optoelectronics - Session Chairs: J. Randall (Zyvex) and S. Matsui (Hyogo) 13:30 Invited 5 Nanoimprint lithography for organic thin film transistors Barbara Stadlober Joanneum Research 13:50 c6 Fabrication of organic TFT arrays on an A4-sized flexible sheet using microcontact printing Hiroshi Fujita Dai Nippon Printing Co., Ltd. -

Etude Des Techniques De Super-Résolution Latérale En Nanoscopie Et Développement D’Un Système Interférométrique Nano-3D Audrey Leong-Hoï
Etude des techniques de super-résolution latérale en nanoscopie et développement d’un système interférométrique nano-3D Audrey Leong-Hoï To cite this version: Audrey Leong-Hoï. Etude des techniques de super-résolution latérale en nanoscopie et développement d’un système interférométrique nano-3D. Micro et nanotechnologies/Microélectronique. Université de Strasbourg, 2016. Français. NNT : 2016STRAD048. tel-02003485 HAL Id: tel-02003485 https://tel.archives-ouvertes.fr/tel-02003485 Submitted on 1 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITÉ DE STRASBOURG ÉCOLE DOCTORALE MATHEMATIQUES, SCIENCES DE L'INFORMATION ET DE L'INGENIEUR (MSII) – ED 269 LABORATOIRE DES SCIENCES DE L'INGENIEUR, DE L'INFORMATIQUE ET DE L'IMAGERIE (ICUBE UMR 7357) THÈSE présentée par : Audrey LEONG-HOI soutenue le : 2 DÉCEMBRE 2016 pour obtenir le grade de : Docteur de l’université de Strasbourg Discipline / Spécialité : Electronique, microélectronique, photonique Étude des techniques de super-résolution latérale en nanoscopie et développement d'un système interférométrique nano-3D THÈSE dirigée par : Dr. MONTGOMERY Paul Directeur de recherche, CNRS, ICube (Strasbourg) Pr. SERIO Bruno Professeur des Universités, Université Paris Ouest, LEME (Paris) RAPPORTEURS : Dr. GORECKI Christophe Directeur de recherche, CNRS, FEMTO-ST (Besançon) Pr. -

Cooperative 4Pi Excitation and Detection Yields Sevenfold Sharper Optical Sections in Live-Cell Microscopy
4146 Biophysical Journal Volume 87 December 2004 4146–4152 Cooperative 4Pi Excitation and Detection Yields Sevenfold Sharper Optical Sections in Live-Cell Microscopy Hilmar Gugel,* Jo¨rg Bewersdorf,y Stefan Jakobs,y Johann Engelhardt,z Rafael Storz,* and Stefan W. Hellyz *Leica Microsystems Heidelberg GmbH, 68165 Mannheim, Germany; yMax Planck Institute for Biophysical Chemistry, Department of NanoBiophotonics, 37070 Go¨ttingen, Germany; and zGerman Cancer Research Center, High Resolution Optical Microscopy Division, 69120 Heidelberg, Germany ABSTRACT Although the addition of just the excitation light field at the focus, or of just the fluorescence field at the detector is sufficient for a three- to fivefold resolution increase in 4Pi-fluorescence microscopy, substantial improvements of its optical properties are achieved by exploiting both effects simultaneously. They encompass not only an additional expansion of the optical bandwidth, but also an amplified transfer of the newly gained spatial frequencies to the image. Here we report on the realization and the imaging properties of this 4Pi microscopy mode of type C that also is the far-field microscope with the hitherto largest aperture. We show that in conjunction with two-photon excitation, the resulting optical transfer function displays a sevenfold improvement of axial three-dimensional resolution over confocal microscopy in aqueous samples, and more importantly, a marked transfer of all frequencies within its inner region of support. The latter is present also without the confocal pinhole. Thus, linear image deconvolution is possible both for confocalized and nonconfocalized live-cell 4Pi imaging. Realized in a state-of-the-art scanning microscope, this approach enables robust three-dimensional imaging of fixed and live cells at ;80 nm axial resolution. -

Simple and Open 4F Koehler Transmitted Illumination System for Low- Cost Microscopic Imaging and Teaching
Simple and open 4f Koehler transmitted illumination system for low- cost microscopic imaging and teaching Jorge Madrid-Wolff1, Manu Forero-Shelton2 1- Department of Biomedical Engineering, Universidad de los Andes, Bogota, Colombia 2- Department of Physics, Universidad de los Andes, Bogota, Colombia [email protected] ORCID: JMW: https://orcid.org/0000-0003-3945-538X MFS: https://orcid.org/0000-0002-7989-0311 Any potential competing interests: NO Funding information: 1) Department of Physics, Universidad de los Andes, Colombia, 2) Colciencias grant 712 “Convocatoria Para Proyectos De Investigación En Ciencias Básicas“ 3) Project termination grant from the Faculty of Sciences, Universidad de los Andes, Colombia. Author contributions: JMW Investigation, Visualization, Writing (Original Draft Preparation) MFS Conceptualization, Funding Acquisition, Methodology, Supervision, Writing(Original Draft Preparation) 1 Title Simple and open 4f Koehler transmitted illumination system for low-cost microscopic imaging and teaching Abstract Koehler transillumination is a powerful imaging method, yet commercial Koehler condensers are difficult to integrate into tabletop systems and make learning the concepts of Koehler illumination difficult. We propose a simple 4f Koehler illumination system that offers advantages with respect to building simplicity, cost and compatibility with tabletop systems, which can be integrated with open source Light Sheet Fluorescence Microscopes (LSFMs). With those applications in mind as well as teaching, we provide -

Dual-Color 4Pi-Confocal Microscopy with 3D-Resolution in the 100 Nm Range
Ultramicroscopy 90 (2002) 207–213 Dual-color 4Pi-confocal microscopy with 3D-resolution in the 100 nm range Hiroshi Kano, Stefan Jakobs, Matthias Nagorni, Stefan W. Hell* High Resolution Optical Microscopy Group, Max-Planck Institute for Biophysical Chemistry, Am Fassberg 11, 37070 Gottin. gen, Germany Received 21 November 2000; received in revised form 2 July 2001 Abstract We report the development of simultaneous two-color channel recordingin 4Pi-confocal microscopy. A marked increase of spatial resolution over confocal microscopy becomes manifested in 4Pi-confocal three-dimensional (3D) data stacks of dual-labeled objects. The fundamentally improved resolution is verified both with densely labeled fluorescence beads as well as with membrane labeled fixed Escherichia coli. The synergistic combination of dual-color 4Pi-confocal recording with image restoration results in dual-color imaging with a 3D resolution in the 100 nm range. r 2002 Elsevier Science B.V. All rights reserved. 1. Introduction the typically 3.5 times sharper main maximum [1]. The side-lobes are removed mathematically by a By providinga fundamental improvement of linear filter, in which case a typical axial resolution axial sectioning, 4Pi-confocal microscopy [1] is a of 130–140 nm is achieved at a two-photon promisingdevelopment in three-dimensional (3D) excitation wavelength of 700–800 nm [3]. Alterna- far-field fluorescence microscopy. The 4Pi-confo- tively, the effective 3D-point spread function cal microscope owes its superior sectioningcap- (PSF) of the 4Pi-confocal microscope is used ability to the coherent addition of the spherical altogether to restore the 4Pi-confocal image data wavefronts produced by two opposingobjective with a non-linear restoration algorithm [4]. -

Two-Photon Excitation and Photoconversion of Eosfp in Dual-Color 4Pi Confocal Microscopy
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biophysical Journal Volume 92 June 2007 4451–4457 4451 Two-Photon Excitation and Photoconversion of EosFP in Dual-Color 4Pi Confocal Microscopy Sergey Ivanchenko,* Sylvia Glaschick,* Carlheinz Ro¨cker,* Franz Oswald,y Jo¨rg Wiedenmann,z and G. Ulrich Nienhaus*§ *Institute of Biophysics, yDepartment of Internal Medicine I, and zInstitute of General Zoology and Endocrinology, University of Ulm, 89069 Ulm, Germany; and §Department of Physics, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801 ABSTRACT Recent years have witnessed enormous advances in fluorescence microscopy instrumentation and fluorescent marker development. 4Pi confocal microscopy with two-photon excitation features excellent optical sectioning in the axial di- rection, with a resolution in the 100 nm range. Here we apply this technique to cellular imaging with EosFP, a photoactivatable autofluorescent protein whose fluorescence emission wavelength can be switched from green (516 nm) to red (581 nm) by irradiation with 400-nm light. We have measured the two-photon excitation spectra and cross sections of the green and the red species as well as the spectral dependence of two-photon conversion. The data reveal that two-photon excitation and photo- activation of the green form of EosFP can be selectively performed by choosing the proper wavelengths. Optical highlighting of small subcellular compartments was shown on HeLa cells expressing EosFP fused to a mitochondrial targeting signal. After three-dimensionally confined two-photon conversion of EosFP within the mitochondrial networks of the cells, the converted re- gions could be resolved in a 3D reconstruction from a dual-color 4Pi image stack. -
Milestone 21: Light Microscopy at the Limit
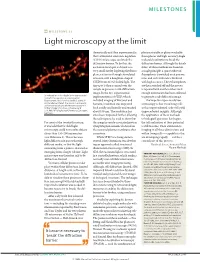
MILESTONES MILESTONE 21 Light microscopy at the limit theoretically and then experimentally, photoactivatable or photoswitchable that a stimulated-emission-depletion fluorophores and high-accuracy single- (STED) microscope can break the molecule localization to break the diffraction barrier. To do this, the diffraction barrier. Although the details excitation focal spot is shrunk to a differ, all three methods are based on very small size by depleting the fluoro- a single principle: a sparse subset of phores at its rim through stimulated fluorophores is switched on at any one emission with a doughnut-shaped time, and each molecule is localized STED beam of red-shifted light. The with high accuracy. These fluorophores tiny spot is then scanned over the are then switched off and the process sample to generate a sub-diffraction is repeated with another subset until image. In the first experimental enough information has been collected Stimulated-emission-depletion image recorded from a living neuron of an organotypical implementation of STED, which to generate a sub-diffraction image. hippocampal slice, showing dendritic spines in included imaging of live yeast and The hope for super-resolution unprecedented detail. The neuron is expressing yellow fluorescent protein. Image courtesy of K. bacteria, resolution was improved microscopy is that visualizing cells Willig, V. Nägerl, N. Urban, T. Bonhoeffer & both axially and laterally and reached at this unprecedented scale will yield S. W. Hell, MPI Biophysical Chemistry, Göttingen, Germany about 100 nm. The resolution has unprecedented insights. Although since been improved further, allowing the application of these methods the technique to be used to show that to biological questions has begun, For most of the twentieth century, the synaptic vesicle-associated protein the full realization of their potential it was held that far-field light synaptotagmin remains clustered on is still to come. -

Accurate 4Pi Single-Molecule Localization Using an Experimental PSF Model
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.18.997163; this version posted March 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Accurate 4Pi single-molecule localization using an experimental PSF model Yiming Li1,2, Elena Buglakova2,3, Yongdeng Zhang4, Jervis Vermal Thevathasan2,5, Joerg Bewersdorf4,6,7,8, Jonas Ries2,* 1Department of Biomedical Engineering, Southern University of Science and Technology, Shenzhen 518055, China 2European Molecular Biology Laboratory, Cell Biology and Biophysics, Heidelberg 69117, Germany 3Moscow Institute of Physics and Technology, Dolgoprudny 141701, Russia 4Department of Cell Biology, Yale School of Medicine, New Haven, CT 06511, USA 5Faculty of Biosciences, Heidelberg University, Heidelberg 69120, Germany 6Kavli Institute for Neuroscience, Yale School of Medicine, New Haven, CT 06511, USA 7Nanobiology Institute, Yale University, West Haven, CT 06516, USA 8Department of Biomedical Engineering, Yale University, New Haven, CT 06511, USA *Correspondence should be addressed to J.R. ([email protected]) Interferometric single-molecule localization microscopy (iPALM, 4Pi-SMS) uses multiphase interferometry to localize single fluorophores and achieves nanometer isotropic resolution in 3D. The current data analysis workflow, however, fails to reach the theoretical resolution limit due to the suboptimal localization algorithm. Here, we develop a method to fit an experimentally derived point spread function (PSF) model to the interference 4Pi-PSF. As the interference phase is not fixed with respect to the shape of the PSF, we decoupled the phase term in the model from the 3D position of the PSF. -

Multiplexed Biomimetic Lipid Membranes on Graphene by Dip-Pen Nanolithography
ARTICLE Received 17 May 2013 | Accepted 10 Sep 2013 | Published 10 Oct 2013 DOI: 10.1038/ncomms3591 OPEN Multiplexed biomimetic lipid membranes on graphene by dip-pen nanolithography Michael Hirtz1,*, Antonios Oikonomou2,*, Thanasis Georgiou3, Harald Fuchs1,4 & Aravind Vijayaraghavan5 The application of graphene in sensor devices depends on the ability to appropriately func- tionalize the pristine graphene. Here we show the direct writing of tailored phospholipid membranes on graphene using dip-pen nanolithography. Phospholipids exhibit higher mobility on graphene compared with the commonly used silicon dioxide substrate, leading to well-spread uniform membranes. Dip-pen nanolithography allows for multiplexed assembly of phospholipid membranes of different functionalities in close proximity to each other. The membranes are stable in aqueous environments and we observe electronic doping of graphene by charged phospholipids. On the basis of these results, we propose phospholipid membranes as a route for non-covalent immobilization of various functional groups on graphene for applications in biosensing and biocatalysis. As a proof of principle, we demonstrate the specific binding of streptavidin to biotin-functionalized membranes. The combination of atomic force microscopy and binding experiments yields a consistent model for the layer organization within phospholipid stacks on graphene. 1 Institute of Nanotechnology (INT) and Karlsruhe Nano Micro Facility (KNMF), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. 2 School of Computer Science and Centre for Mesoscience and Nanotechnology, The University of Manchester, Manchester M13 9PL, UK. 3 School of Physics and Astronomy, The University of Manchester, Manchester M13 9PL, UK. 4 Physical Institute and Center for Nanotechnology (CeNTech), University of Mu¨nster, Wilhelm-Klemm-Str. -

Bright-Field Microscopy
Chapter 4 Bright-Field Microscopy Our naked eye is unable to resolve two objects that are sep- the human eye to resolve two dots that are closer than 70 µ m arated by less than 70 µ m. Perhaps we are fortunate that, from each other. The retina contains approximately 120 without a microscope, our eyes are unable to resolve small million rods and 8 million cones packed into a single layer. distances. John Locke (1690) wrote in An Essay Concerning In the region of highest resolution, known as the fovea, Human Understanding , the cones are packed so tightly that they are only 2 µ m apart. We are able, by our senses, to know and distinguish things.... Still, this distance between the cones limits the resolving if that most instructive of our senses, seeing, were in any man a power of our eyes. If a point of light originating from one thousand or a hundred thousand times more acute than it is by object falls on only one cone, that object will appear as a the best microscope, things several millions of times less than the point. If light from two objects that are close together fall smallest object of his sight now would then be visible to his on one cone, the two objects will appear as one. When naked eyes, and so he would come nearer to the discovery of the texture and motion of the minute parts of corporeal things; and in light from two points fall on two separate cones separated many of them, probably get ideas of their internal constitutions: by a third cone, the two points can be clearly resolved.