"Ethanolamines and Propanolamines," In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Development of a Process for N-Butanol Recovery from Abe
937 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 74, 2019 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza Copyright © 2019, AIDIC Servizi S.r.l. ISBN 978-88-95608-71-6; ISSN 2283-9216 DOI: 10.3303/CET1974157 Development of a Process for N-Butanol Recovery from Abe Wastewater Streams by Membrane Technology * Marco Stoller , Marco Bravi, Paola Russo, Roberto Bubbico, Barbara Mazzarotta Sapienza University of Rome, Dept. of Chemical Materials Environmental Engineering, Via Eudossiana 18, 00184 Rome, Italy [email protected] The aceton-butyl-ethanolic fermentation process (ABE) is a biotechnological process that leads to the production of acetone, n-butanol and ethanol (ABE compounds) from glucose sources and amides by use of certain biomasses. The process was developed initially during the middle of the last century and suffers from decline due to the greater petrochemical production of products and the lowering of the costs of the sector. Nowadays, the ABE process is regaining great interest because the fraction with the highest concentration, i.e. n-butanol, is an excellent constituent for biofuels. The ABE process has been optimized over time to obtain maximum yields of n-butanol, but the problem of separating and concentrating the butanol in the outlet stream of the ABE process persists. To allow an adequate use, often distillation by use of more columns is required. Moreover, the contained biomasses and suspended solids, in high quantity, must be eliminated, leading to overall high treatment costs. This work will report the main idea and some preliminary experimental results for the development and application of a process based on membrane technologies, to separate and concentrate the butanol from ABE process streams to sensibly reduce the difficulty to perform a final distillation. -

Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Rutledge, James Edward, "Evaluation of Azeotropic Dehydration for the Preservation of Shrimp." (1969). LSU Historical Dissertations and Theses. 1689. https://digitalcommons.lsu.edu/gradschool_disstheses/1689 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 70-9089 microfilmed exactly as received RUTLEDGE, James Edward, 1941- EVALUATION OF AZEOTROPIC DEHYDRATION FOR THE PRESERVATION OF SHRIMP. The Louisiana State University and Agricultural and Mechanical College, PhJD., 1969 Food Technology University Microfilms, Inc., Ann Arbor, Michigan Evaluation of Azeotropic Dehydration for the Preservation of Shrimp A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Food Science and Technology by James Edward Rutledge B.S., Texas A&M University, 1963 M.S., Texas A&M University, 1966 August, 1969 ACKNOWLEDGMENT The author wishes to express his sincere appreciation to his major professor, Dr, Fred H. Hoskins, for the guidance which he supplied not only in respect to this dissertation but also in regard to the author’s graduate career at Louisiana State University, Gratitude is also extended to Dr. -
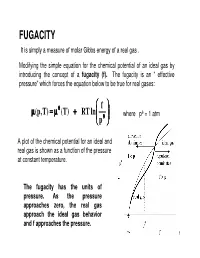
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

779 Part 770—Interpretations
Pt. 770 15 CFR Ch. VII (1–1–21 Edition) in the item that qualitatively affect the per- roller bearings and parts). This applies formance of the U.S. and foreign items; to separate shipments of anti-friction (vi) Evidence of the interchangeability of bearings or bearing systems and anti- U.S. and foreign items; friction bearings or bearing systems (vii) Patent descriptions for the U.S. and foreign items; shipped with machinery or equipment (viii) Evidence that the U.S. and foreign for which they are intended to be used items meet a published industry, national, or as spares or replacement parts. international standard; (2) An anti-friction bearing or bear- (ix) A report or eyewitness account, by ing system physically incorporated in a deposition or otherwise, of the foreign item’s segment of a machine or in a complete operation; machine prior to shipment loses its (x) Evidence concerning the foreign manu- identity as a bearing. In this scenario, facturers’ corporate reputation; (xi) Comparison of the U.S. and foreign end the machine or segment of machinery item(s) made from a specific commodity, containing the bearing is the item sub- tool(s), device(s), or technical data; or ject to export control requirements. (xii) Evidence of the reputation of the for- (3) An anti-friction bearing or bear- eign item including, if possible, information ing system not incorporated in a seg- on maintenance, repair, performance, and ment of a machine prior to shipment, other pertinent factors. but shipped as a component of a com- plete unassembled (knocked-down) ma- SUPPLEMENT NO. -

Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard
Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude To cite this version: Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas. Journal of Physical Chemistry A, American Chemical Society, 2017, 121 (17), pp.3254-3262. 10.1021/acs.jpca.7b01238. hal-01708219 HAL Id: hal-01708219 https://hal.archives-ouvertes.fr/hal-01708219 Submitted on 13 Feb 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta†, Baptiste Sirjean†, Laurent Verdier‡, René Fournet†, Pierre-Alexandre Glaude†,* †Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, 1 rue Grandville BP 20451 54001 Nancy Cedex, France ‡DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France *corresponding author: [email protected] Abstract The destruction of stockpiles or unexploded ammunitions of nitrogen mustard (tris (2- chloroethyl) amine, HN-3) requires the development of safe processes. -

Monoethanolamine Diethanolamine Triethanolamine DSA9781.Qxd 1/31/03 10:21 AM Page 2
DSA9781.qxd 1/31/03 10:21 AM Page 1 ETHANOLAMINES Monoethanolamine Diethanolamine Triethanolamine DSA9781.qxd 1/31/03 10:21 AM Page 2 CONTENTS Introduction ...............................................................................................................................2 Ethanolamine Applications.........................................................................................................3 Gas Sweetening ..................................................................................................................3 Detergents, Specialty Cleaners, Personal Care Products.......................................................4 Textiles.................................................................................................................................4 Metalworking ......................................................................................................................5 Other Applications...............................................................................................................5 Ethanolamine Physical Properties ...............................................................................................6 Typical Physical Properties ....................................................................................................6 Vapor Pressure of Ethanolamines (Figure 1).........................................................................7 Heat of Vaporization of Ethanolamines (Figure 2)................................................................7 Specific -

Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas
processes Article Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas Usman Shoukat , Diego D. D. Pinto and Hanna K. Knuutila * Department of Chemical Engineering, Norwegian University of Science and Technology (NTNU), 7491 Trondheim, Norway; [email protected] (U.S.); [email protected] (D.D.D.P.) * Correspondence: [email protected] Received: 8 February 2019; Accepted: 8 March 2019; Published: 15 March 2019 Abstract: Various novel amine solutions both in aqueous and non-aqueous [monoethylene glycol (MEG)/triethylene glycol(TEG)] forms have been studied for hydrogen sulfide (H2S) absorption. The study was conducted in a custom build experimental setup at temperatures relevant to subsea operation conditions and atmospheric pressure. Liquid phase absorbed H2S, and amine concentrations were measured analytically to calculate H2S loading (mole of H2S/mole of amine). Maximum achieved H2S loadings as the function of pKa, gas partial pressure, temperature and amine concentration are presented. Effects of solvent type on absorbed H2S have also been discussed. Several new solvents showed higher H2S loading as compared to aqueous N-Methyldiethanolamine (MDEA) solution which is the current industrial benchmark compound for selective H2S removal in natural gas sweetening process. Keywords: H2S absorption; amine solutions; glycols; desulfurization; aqueous and non-aqueous solutions 1. Introduction Natural gas is considered one of the cleanest forms of fossil fuel. Its usage in industrial processes and human activities is increasing worldwide, providing 23.4% of total world energy requirement in 2017 [1]. Natural gas is half of the price of crude oil and produces 29% less carbon dioxide than oil per unit of energy output [2]. -

By a 965% ATT'ys
July 19, 1960 A. WATZL ETAL 2,945,788 PROCESS FOR THE PURIFICATION OF DIMETHYLTEREPHTHALATE Filed Nov. 19, l956 AZEOTROPE DISTILLATE CONDENSER CONDENSED WACUUM DISTILLATE DRY DISTILLATION MXTURE N2 COLUMN MPURE - SOD WACUUMFILTER ETHYLENE DMETHYL DMETHYL GLYCOL TEREPHTHALATE TEREPHTHALATE ETHYLENE GLYCOL INVENTORS: ANTON WATZL by aERHARD 965% SIGGEL ATT'YS 2,945,788 Patented July 19, 1960 2 phthalate is immediately suitable for reesterification or Subsequent polycondensation to polyethylene terephthal 2,945,788 ate. There are gained polycondensates of high degree of PROCESS FOR THE PURIFICATION OF viscosity with K values from 50 to 57. DMETHYLTEREPHTHALATE The best mode contemplated for practicing the inven Anton Watz, Kleinwallstadt (Ufr), and Erhard Siggel, tion involves the use of ethylene glycol as the aliphatic Laudenbach (Main), Germany, assignors to Vereinigte glycol. The following is a specific illustration thereof. Glanzstoff-Fabriken A.G., Wuppertal-Elberfeld, Ger Example many Thirty grams of crude dimethylterephthalate are mixed Filed Nov.19, 1956, Ser. No. 622,778 in a flask with 270 grams of ethylene glycol and azeo tropically distilled with the introduction of dry nitrogen 4 Claims. (C. 202-42) in a column of 30 cm. at about 44 Torr (1 Torr equals 1 mm. Hg). The azeotrope goes over at about 120° C. i This invention, in general, relates to production of into a cooled condenser. The dimethylterephthalate dimethylterephthalate and more particularly to the puri 5 separated from the glycol by vacuum filtering can be fication thereof. used immediately for reesterification or for polyconden The purification of dimethylterephthalate can be car sation. This process is illustrated in the flow sheet of ried out either by distillation or by recrystallization from the accompanying drawing. -

Heterogeneous Azeotropic Distillation
PROSIMPLUS APPLICATION EXAMPLE HETEROGENEOUS AZEOTROPIC DISTILLATION EXAMPLE PURPOSE This example illustrates a high purity separation process of an azeotropic mixture (ethanol-water) through heterogeneous azeotropic distillation. This process includes distillation columns. Additionally these rigorous multi- stage separation modules are part of a recycling stream, demonstrating the efficiency of ProSimPlus convergence methods. Specifications are set on output streams in order to insure the required purity. This example illustrates the way to set "non-standard" specifications in the multi-stage separation modules. Three phase calculations (vapor-liquid- liquid) are done with the taken into account of possible liquid phase splitting. ACCESS Free-Internet Restricted to ProSim clients Restricted Confidential CORRESPONDING PROSIMPLUS FILE PSPS_EX_EN-Heterogeneous-Azeotropic-Distillation.pmp3 . Reader is reminded that this use case is only an example and should not be used for other purposes. Although this example is based on actual case it may not be considered as typical nor are the data used always the most accurate available. ProSim shall have no responsibility or liability for damages arising out of or related to the use of the results of calculations based on this example. Copyright © 2009 ProSim, Labège, France - All rights reserved www.prosim.net Heterogeneous azeotropic distillation Version: March, 2009 Page: 2 / 12 TABLE OF CONTENTS 1. PROCESS MODELING 3 1.1. Process description 3 1.2. Process flowsheet 5 1.3. Specifications 6 1.4. Components 6 1.5. Thermodynamic model 7 1.6. Operating conditions 7 1.7. "Hints and Tips" 9 2. RESULTS 9 2.1. Comments on results 9 2.2. Mass and energy balances 10 2.3. -

TR-449: Triethanolamine (CASRN 102-71-6) in F344 Rats and B6c3f1mice (Inhalation Studies)
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF TRIETHANOLAMINE (CAS NO. 102-71-6) IN F344/N RATS AND B6C3F1 MICE (DERMAL STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 November 1999 NTP TR 449 NIH Publication No. 00-3365 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Technical Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

Ethanolamines Storage Guide Dow Manufactures Ethanolamines for A
DSA9782.qxd 1/29/03 2:34 PM Page 1 DSA9782.qxd 1/29/03 2:34 PM Page 2 DSA9782.qxd 1/29/03 2:34 PM Page 3 Contents PAGE Introduction 2 Product Characteristics 3 Occupational Health 3 Reactivity 3 Oxidation 4 Liquid Thermal Stability 4 Materials of Construction 5 Pure Ethanolamines 5 Aqueous Ethanolamines 6 Gaskets and Elastomers 7 Transfer Hose 8 Preparation for Service 9 Thermal Insulation Materials 10 Typical Storage System 11 Tank and Line Heating 11 Drum Thawing 11 Special Considerations 14 Vent Freezing 14 Color Buildup in Traced Pipelines 14 Thermal Relief for Traced Lines 14 Product Unloading 15 Unloading System 15 Shipping Vessel Descriptions 16 General Unloading Procedure 17 Product Handling 18 Personal Protective Equipment 18 Firefighting 18 Equipment Cleanup 18 Product Shipment 19 Environmental Considerations 19 Product Safety 20 1 DSA9782.qxd 1/29/03 2:34 PM Page 4 Ethanolamines Storage and Handling The Dow Chemical Company manufactures high-quality ethanolamines for a wide variety of end uses. Proper storage and handling will help maintain the high quality of these products as they are delivered to you. This will enhance your ability to use these products safely in your processes and maximize performance in your finished products. Ethanolamines have unique reactivity and solvent properties which make them useful as intermediates for a wide variety of applications. As a group, they are viscous, water-soluble liquids. In their pure, as-delivered state, these materials are chemically stable and are not corrosive to the proper containers. Ethanolamines can freeze at ambient temperatures.