Separation of Dimethyl Carbonate and Methanol Mixture by Pervaporation Using Hybsi Ceramic Membrane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Development of a Process for N-Butanol Recovery from Abe
937 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 74, 2019 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza Copyright © 2019, AIDIC Servizi S.r.l. ISBN 978-88-95608-71-6; ISSN 2283-9216 DOI: 10.3303/CET1974157 Development of a Process for N-Butanol Recovery from Abe Wastewater Streams by Membrane Technology * Marco Stoller , Marco Bravi, Paola Russo, Roberto Bubbico, Barbara Mazzarotta Sapienza University of Rome, Dept. of Chemical Materials Environmental Engineering, Via Eudossiana 18, 00184 Rome, Italy [email protected] The aceton-butyl-ethanolic fermentation process (ABE) is a biotechnological process that leads to the production of acetone, n-butanol and ethanol (ABE compounds) from glucose sources and amides by use of certain biomasses. The process was developed initially during the middle of the last century and suffers from decline due to the greater petrochemical production of products and the lowering of the costs of the sector. Nowadays, the ABE process is regaining great interest because the fraction with the highest concentration, i.e. n-butanol, is an excellent constituent for biofuels. The ABE process has been optimized over time to obtain maximum yields of n-butanol, but the problem of separating and concentrating the butanol in the outlet stream of the ABE process persists. To allow an adequate use, often distillation by use of more columns is required. Moreover, the contained biomasses and suspended solids, in high quantity, must be eliminated, leading to overall high treatment costs. This work will report the main idea and some preliminary experimental results for the development and application of a process based on membrane technologies, to separate and concentrate the butanol from ABE process streams to sensibly reduce the difficulty to perform a final distillation. -

International Journal of Engineering Sciences
[Kanse*, 4.(12): December, 2015] ISSN: 2277-9655 (I2OR), Publication Impact Factor: 3.785 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY A REVIEW OF PERVAPORATION MEMBRANE SYSTEM FOR THE SEPARATION OF ETHANOL/WATER (AZEOTROPIC MIXTURE) Nitin G. Kanse*, Dr. S. D. Dawande * Research Scholar, Department of Chemical Engineering Laxminarayan Institute of Technology, Nagpur. ABSTRACT Membrane separation process has become one of the emerging technologies that undergo a rapid growth for the past few decades. Pervaporation is the one of the membrane separation processes that have gained increasing interest in the chemical and allied industries. Pervaporation has significant advantages in separation of azeotropic systems where traditional distillation can recover pure solvents with the use of entrainers, which then must be removed using an additional separation step. Pervaporation can be used to break the azeotrope, as its mechanism of separation is quite different to that of distillation. This review presents the separation of Azeotropic mixture such as ethanol-water by using Pervaporation. The fundamental aspects of Pervaporation over different types of membrane are revived and compared. The focus of this review is on separation of azeotropic mixture by using different membrane and effect of various parameters on Pervaporation. KEYWORDS: Pervaporation, Azeotropic mixture, Membrane, selectivity. INTRODUCTION Pervaporation is a new membrane technique which is used to separate a liquid mixture by partly vaporizing it through a nonporous permselective membrane. The feed mixture is allowed to flow along one side of the membrane and a fraction of it (the ‘permeate’) is evolved in the vapor state from the opposite side, which is kept under vacuum by continuous pumping Membrane separation processes offer many advantages over existing separation processes such as higher selectivity, lower energy consumption, moderate cost to performance ratio and compact with modular design [12, 19]. -

Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Rutledge, James Edward, "Evaluation of Azeotropic Dehydration for the Preservation of Shrimp." (1969). LSU Historical Dissertations and Theses. 1689. https://digitalcommons.lsu.edu/gradschool_disstheses/1689 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 70-9089 microfilmed exactly as received RUTLEDGE, James Edward, 1941- EVALUATION OF AZEOTROPIC DEHYDRATION FOR THE PRESERVATION OF SHRIMP. The Louisiana State University and Agricultural and Mechanical College, PhJD., 1969 Food Technology University Microfilms, Inc., Ann Arbor, Michigan Evaluation of Azeotropic Dehydration for the Preservation of Shrimp A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Food Science and Technology by James Edward Rutledge B.S., Texas A&M University, 1963 M.S., Texas A&M University, 1966 August, 1969 ACKNOWLEDGMENT The author wishes to express his sincere appreciation to his major professor, Dr, Fred H. Hoskins, for the guidance which he supplied not only in respect to this dissertation but also in regard to the author’s graduate career at Louisiana State University, Gratitude is also extended to Dr. -

Kinetic Modeling and Mechanistic Investigations of Transesterification of Propylene Carbonate with Methanol Over Fe-Mn Double Metal Cyanide Catalyst
Reaction Chemistry & Engineering Kinetic Modeling and Mechanistic Investigations of Transesterification of Propylene Carbonate with Methanol over Fe-Mn Double Metal Cyanide Catalyst Journal: Reaction Chemistry & Engineering Manuscript ID RE-ART-09-2019-000372.R1 Article Type: Paper Date Submitted by the 21-Oct-2019 Author: Complete List of Authors: Song, Ziwei; Yanshan University, Subramaniam, Bala; University of Kansas, Center for Environmentally Beneficial Catalysis Chaudhari, Raghunath; University of Kansas, Chemical & Petroleum Engineering Page 1 of 23 Reaction Chemistry & Engineering Kinetic Modeling and Mechanistic Investigations of Transesterification of Propylene Carbonate with Methanol over Fe-Mn Double Metal Cyanide Catalyst Ziwei Song, a,b,c Bala Subramaniam, a,b and Raghunath V. Chaudhari* a,b aCenter for Environmentally Beneficial Catalysis, University of Kansas, 1501 Wakarusa Dr. Lawrence, KS 66047, United States bDepartment of Chemical & Petroleum Engineering, University of Kansas, 1503 W 15th St. Lawrence, KS 66045, United States cDepartment of Chemical Engineering, College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China *Corresponding author: [email protected] KEYWORDS: double metal cyanide, transesterification, dimethyl carbonate, microkinetic modeling 1 Reaction Chemistry & Engineering Page 2 of 23 Abstract Kinetic modeling of transesterification of propylene carbonate with methanol using Fe-Mn double metal cyanide catalyst has been investigated based on experimental data obtained under kinetically controlled conditions in a batch slurry reactor in the 140-200 °C range. A simple two-step power law model was found to represent the experimental data well. In addition, a detailed kinetic model based on a molecular level description of the reaction mechanism is also evaluated to provide better insight into the reaction mechanism. -

Application of Pervaporation and Vapor Permeation in Environmental Protection
Polish Journal of Environmental Studies Vol. 9, No. 1 (2000), 13-26 Application of Pervaporation and Vapor Permeation in Environmental Protection W. Kujawski Faculty of Chemistry, Nicholas Copernicus University, ul. Gagarina 7, 87-100 Toruń, Poland e-mail: [email protected] Abstract A review concerning pervaporation and vapor permeation - membrane separation techniques used to separate liquid mixtures, is presented. Examples of polymers for membrane preparation as well as perform- ance parameters of pervaporation and vapor permeation membranes are described. The second part of the paper presents applications of pervaporation and vapor permeation in environmental protection. At the present, liquid product mixtures must fulfill high purity requirements as well as effluents; there- fore, they have to be concentrated or reconditioned. In the process of product-integrated environmental protection, liquid substances should be separated specifically from the mainstream, either to save raw materials, to prevent or to minimize the disposal of effluents, or to recycle by-products. Such completely or partly soluble fluid mixtures can be separated with membrane methods. Pervaporation and vapor per- meation as the most well-known membrane processes for the separation of liquid and vapor mixtures allow a variety of possible application areas: i) dewatering of organic fluids like alcohols, ketones, ethers etc.; ii) separation of mixtures from narrow boiling temperatures to constant (azeotrop) boiling temperatures; iii) removal of organic pollutants from water and air streams; iv) separation of fermentation products; v) separation of organic-organic liquid mixtures. Keywords: pervaporation, vapor permeation, polymers, membrane methods, environmental protection Introduction ous organics from contaminated water in applications Most industrial scale separation processes are based such as groundwater remediation. -

Dimethyl Carbonate
SAN JOAQUIN VALLEY UNIFIED AIR POLLUTION CONTROL DISTRICT Appendix B: OEHHA Final Revised Assessment November 18, 2010 APPENDIX B OEHHA FINAL REVISED HEALTH ASSESSMENT FOR DIMETHYL CARBONATE November 18, 2010 Draft Staff Report with Appendices for Draft Amendments to Rule 1020 SAN JOAQUIN VALLEY UNIFIED AIR POLLUTION CONTROL DISTRICT Appendix B: OEHHA Final Revised Assessment November 18, 2010 This page intentionally blank. B-2 Draft Staff Report with Appendices for Draft Amendments to Rule 1020 Appendix B: OEHHA Assessment November 18, 2010 Office of Environmental Health Hazard Assessment Joan E. Denton, Ph.D., Director Headquarters ••• 1001 I Street ••• Sacramento, California 95814 Mailing Address: P.O. Box 4010 ••• Sacramento, California 95812-4010 Oakland Office ••• Mailing Address: 1515 Clay Street, 16th Floor ••• Oakland, California 94612 Linda S. Adams Arnold Schwarzenegger Secretary for Environmental Protectiony Governor M E M O R A N D U M TO: Richard Corey, Chief Research and Economic Studies Branch Research Division Air Resources Board FROM: Melanie A. Marty, Ph.D., Chief Air Toxicology and Epidemiology Branch DATE: December 8, 2009 SUBJECT: REVISED ASSESSMENT OF HEALTH EFFECTS OF EXPOSURE TO DIMETHYL CARBONATE, A CHEMICAL PETITIONED FOR EXEMPTION FROM VOC RULES Recently the Research Division sent the Office of Environmental Health Hazard Assessment (OEHHA) an application for VOC Exempt Status in the State of California for Dimethyl Carbonate. This was submitted by Kowa Corporation, who propose use of from 2 to possibly 5 million pounds of dimethyl carbonate per year as a niche solvent in California, if dimethyl carbonate is exempted from VOC regulations. In response to a request from the Division, OEHHA recently provided you a review of the health effects of dimethyl carbonate. -
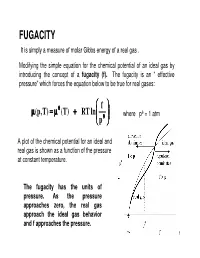
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion
Int. J. Mol. Sci. 2010, 11, 1343-1351; doi:10.3390/ijms11041343 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion Ahmed Aouissi *, Zeid Abdullah Al-Othman and Amro Al-Amro Department of Chemistry, King Saud University, P.O. Box 2455, Riyadh-11451, Saudi Arabia * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 22 January 2010; in revised form: 9 March 2010 / Accepted: 29 March 2010 / Published: 31 March 2010 Abstract: The reactivity of Co1.5PW12O40 in the direct synthesis of dimethyl carbonate (DMC) from CO2 and CH3OH was investigated. The synthesized catalyst has been characterized by means of FTIR, XRD, TG, and DTA and tested in gas phase under atmospheric pressure. The effects of the reaction temperature, time on stream, and methanol weight hourly space velocity (MWHSV) on the conversion and DMC selectivity were investigated. The highest conversion (7.6%) and highest DMC selectivity (86.5%) were obtained at the lowest temperature used (200 °C). Increasing the space velocity MWHSV increased the selectivity of DMC, but decreased the conversion. A gain of 18.4% of DMC selectivity was obtained when the MWHSV was increased from 0.65 h-1 to 3.2 h-1. Keywords: heteropolyanion; Keggin structure; methanol; dimethyl carbonate; direct synthesis; carbon dioxide 1. Introduction Dimethyl carbonate (DMC) has drawn much attention in recent years as an environmentally friendly versatile intermediate. It has been used as a good solvent [1], an alkylation agent [2], and a substitute for highly toxic phosgene and dimethyl sulfate in many chemical processes [3,4]. -

A Convenient and Safe O-Methylation of Flavonoids with Dimethyl Carbonate (DMC)
Molecules 2011, 16, 1418-1425; doi:10.3390/molecules16021418 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article A Convenient and Safe O-Methylation of Flavonoids with Dimethyl Carbonate (DMC) Roberta Bernini *, Fernanda Crisante and Maria Cristina Ginnasi Dipartimento di Agrobiologia e Agrochimica, Università degli Studi della Tuscia, Via S. Camillo De Lellis, 01100 Viterbo, Italy * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 16 November 2010; in revised form: 23 January 2011 / Accepted: 27 January 2011 / Published: 9 February 2011 Abstract: Dietary flavonoids exhibit beneficial health effects. Several epidemiological studies have focused on their biological activities, including antioxidant, antibacterial, antiviral, anti-inflammatory and cardiovascular properties. More recently, these compounds have shown to be promising cancer chemopreventive agents in cell culture studies. In particular, O-methylated flavonoids exhibited a superior anticancer activity than the corresponding hydroxylated derivatives being more resistant to the hepatic metabolism and showing a higher intestinal absorption. In this communication we describe a convenient and efficient procedure in order to prepare a large panel of mono- and dimethylated flavonoids by using dimethyl carbonate (DMC), an ecofriendly and non toxic chemical, which plays the role of both solvent and reagent. In order to promote the methylation reaction under mild and practical conditions, 1,8-diazabicyclo[5.4.0]undec-7- ene (DBU) was added in the solution; methylated flavonoids were isolated in high yields and with a high degree of purity. This methylation protocol avoids the use of hazardous and high toxic reagents (diazomethane, dimethyl sulfate, methyl iodide). Keywords: methylated flavonoids; ecofriendly methylation reaction; dimethyl carbonate (DMC); green chemistry 1. -

Recent Progress in Phosgene-Free Methods for Synthesis of Dimethyl Carbonate*
Pure Appl. Chem., Vol. 84, No. 3, pp. 603–620, 2012. http://dx.doi.org/10.1351/PAC-CON-11-06-02 © 2011 IUPAC, Publication date (Web): 22 September 2011 Recent progress in phosgene-free methods for synthesis of dimethyl carbonate* Weicai Peng1, Ning Zhao1, Fukui Xiao1, Wei Wei1,‡, and Yuhan Sun1,2,‡ 1State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China; 2Low Carbon Conversion Center, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201203, China Abstract: Dimethyl carbonate (DMC) is considered as an environmentally benign chemical due to negligible ecotoxicity, low bioaccumulation, and low persistence. However, the tradi- tional process of DMC synthesis via phosgene and methanol is limited in industry owing to the toxic raw material involved. Thus, environmentally friendly phosgene-free processes for DMC production have been proposed and developed in the past decades. Until now, the alter- natives appear to be the oxidative carbonylation of methanol, the transesterification of pro - pylene or ethylene carbonate (PC or EC), the methanolysis of urea, and the direct synthesis of DMC from CO2 with methanol. In this review, we present some recent developments of these phosgene-free approaches and their prospects for industrialization. Keywords: carbon dioxide; dimethyl carbonate synthesis; oxidative carbonylation; phosgene- free; transesterification; urea. INTRODUCTION Along with the global spread of sustainable development strategy, the chemical synthesis processes and materials endangering humans and the environment would be gradually restricted. The “clean produc- tion process” and “green chemicals” will be the developmental direction for the modern chemical indus- try, and the production and chemical utilization of dimethyl carbonate (DMC) are closely concerted by this trend. -

Propionate Dihydrate
(19) & (11) EP 2 247 572 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 241/02 (2006.01) C07C 243/40 (2006.01) 03.08.2011 Bulletin 2011/31 (86) International application number: (21) Application number: 09713008.2 PCT/EP2009/051996 (22) Date of filing: 19.02.2009 (87) International publication number: WO 2009/103773 (27.08.2009 Gazette 2009/35) (54) A ONE-POT PROCESS FOR PREPARING 3-(2,2,2-TRIMETHYLHYDRAZINIUM)PROPIONATE DIHYDRATE EIN-TOPF-VERFAHREN ZUR HERSTELLUNG VON 3-(2,2,2-TRIMETHYLHYDRAZINIUM) PROPIONAT-DIHYDRAT PROCÉDÉ DE PRÉPARATION EN ENCEINTE UNIQUE DE DIHYDRATE DE 3-(2,2,2- TRIMÉTHYLHYDRAZINIUM)-PROPIONATE (84) Designated Contracting States: • OSVALDS, Pugovics AT BE BG CH CY CZ DE DK EE ES FI FR GB GR LV-1004 Riga (LV) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • CERNOBROVIJS, Aleksandrs PT RO SE SI SK TR LV-2114 Olaine (LV) Designated Extension States: • IEVINA, Agnija AL BA RS LV-1004 Riga (LV) • LEBEDEVS, Antons (30) Priority: 19.02.2008 LV 080022 LV-3600 Ventspils (LV) 19.02.2008 LV 080023 (56) References cited: (43) Date of publication of application: WO-A-2005/012233 US-A- 4 481 218 10.11.2010 Bulletin 2010/45 • GILLER S.A. ET AL,.: CHEMISTRY OF (73) Proprietor: Grindeks, a joint stock company HETEROCYCLIC COMPOUNDS, vol. 11, no. 12, Riga 1057 (LV) 1975, pages 1378-1382, XP002534780 (72) Inventors: • KALVINS, Ivars LV-15052 Ikskile (LV) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations.