Biotic Interactions Mediate the Influence of Bird Colonies on Vegetation and Soil Chemistry at Aggregation Sites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Communally Nesting Migratory Birds Create Ecological Hot-Spots in Tropical Australia
RESEARCH ARTICLE Communally Nesting Migratory Birds Create Ecological Hot-Spots in Tropical Australia Daniel J. D. Natusch1,2*, Jessica A. Lyons2, Gregory Brown1, Richard Shine1 1 School of Life and Environmental Sciences, University of Sydney, Sydney, New South Wales, Australia, 2 Resource Evaluation and Development, Bamaga, Queensland, Australia * [email protected] Abstract Large numbers of metallic starlings (Aplonis metallica) migrate annually from New Guinea to the rainforests of tropical Australia, where they nest communally in single emergent trees a11111 (up to 1,000 birds). These aggregations create dense and species-rich faunal “hot-spots”, attracting a diverse assemblage of local consumers that utilise this seasonal resource. The starlings nested primarily in poison-dart trees (Antiaris toxicaria) near the rainforest-wood- land boundary. Surveys underneath these colonies revealed that bird-derived nutrients massively increased densities of soil invertebrates and mammals (primarily wild pigs) beneath trees, year-round. Flying invertebrates, nocturnal birds, reptiles, and amphibians OPEN ACCESS congregated beneath the trees when starlings were nesting (the wet-season). Diurnal birds (primarily cockatoos and bush turkeys) aggregated beneath the trees during the dry-season Citation: Natusch DJD, Lyons JA, Brown G, Shine R (2016) Communally Nesting Migratory Birds Create to utilise residual nutrients when the starlings were not nesting. The abundance of several Ecological Hot-Spots in Tropical Australia. PLoS ONE taxa was considerably higher (to > 1000-fold) under colony trees than under nearby trees. 11(10): e0162651. doi:10.1371/journal.pone.0162651 The system strikingly resembles utilisation of bird nesting colonies by predators in other Editor: Maura (Gee) Geraldine Chapman, University parts of the world but this spectacular system has never been described, emphasizing the of Sydney, AUSTRALIA continuing need for detailed natural-history studies in tropical Australia. -

Varanus Doreanus) in Australia
BIAWAK Journal of Varanid Biology and Husbandry Volume 11 Number 1 ISSN: 1936-296X On the Cover: Varanus douarrha The individuals depicted on the cover and inset of this issue represent a recently redescribed species of monitor lizard, Varanus douarrha (Lesson, 1830), which origi- nates from New Ireland, in the Bismark Archipelago of Papua New Guinea. Although originally discovered and described by René Lesson in 1830, the holotype was lost on its way to France when the ship it was traveling on became shipwrecked at the Cape of Good Hope. Since then, without a holotype for comparitive studies, it has been assumed that the monitors on New Ireland repre- sented V. indicus or V. finschi. Recent field investiga- tions by Valter Weijola in New Ireland and the Bismark Archipelago and phylogenetic analyses of recently col- lected specimens have reaffirmed Lesson’s original clas- sification of this animal as a distinct species. The V. douarrha depicted here were photographed by Valter Weijola on 17 July and 9 August 2012 near Fis- soa on the northern coast of New Ireland. Both individu- als were found basking in coconut groves close to the beach. Reference: Weijola, V., F. Kraus, V. Vahtera, C. Lindqvist & S.C. Donnellan. 2017. Reinstatement of Varanus douarrha Lesson, 1830 as a valid species with comments on the zoogeography of monitor lizards (Squamata: Varanidae) in the Bismarck Archipelago, Papua New Guinea. Australian Journal of Zoology 64(6): 434–451. BIAWAK Journal of Varanid Biology and Husbandry Editor Editorial Review ROBERT W. MENDYK BERND EIDENMÜLLER Department of Herpetology Frankfurt, DE Smithsonian National Zoological Park [email protected] 3001 Connecticut Avenue NW Washington, DC 20008, US RUSTON W. -

A Study Investigating the Biodiversity Value of a Native Timber Plantation Landscape in Papua New Guinea
ResearchOnline@JCU This file is part of the following reference: Pryde, Elizabeth Clare (2014) Tropical production landscapes and conservation: a study investigating the biodiversity value of a native timber plantation landscape in Papua New Guinea. PhD thesis, James Cook University. Access to this file is available from: http://researchonline.jcu.edu.au/43784/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://researchonline.jcu.edu.au/43784/ Tropical production landscapes and conservation: a study investigating the biodiversity value of a native timber plantation landscape in Papua New Guinea Thesis submitted by Elizabeth Clare Pryde B.Sc.Hons. (University of Melbourne) On 29th August 2014 For the Degree of Doctor of Philosophy In the College of Marine and Environmental Sciences James Cook University, Cairns To my magical daughter Amelia STATEMENT OF THE CONTRIBUTION OF OTHERS Research funding • The Australian Pacific Science Foundation (APSF) • Oregon State Zoo • Skyrail Rainforest Foundation • Wentworth Group of Concerned Scientists • James Cook University, Australia Stipend • Australian Postgraduate Award Supervision • Professor Steve Turton • Dr James Moloney Study design and analytical support • Dr John Kanowski (vegetation data collection) • Guy Dutson (bird data collection) • Dr Dale Nimmo (Chapter -

WVCP Bird Paper
Mongabay.com Open Access Journal - Tropical Conservation Science Vol.4 (3):317-348, 2011 Research Article Bird communities of the lower Waria Valley, Morobe Province, Papua New Guinea: a comparison between habitat types 1* 2,3 1,4 Jeff Dawson , Craig Turner , Oscar Pileng , Andrew Farmer1, Cara McGary1, Chris Walsh1, Alexia Tamblyn2 and Cossey Yosi5 1Coral Cay Conservation, 1st Floor Block, 1 Elizabeth House, 39 York Road, London SW1 7NQ UK 2Previous address: Jaquelin Fisher Associates, 4 Yukon Road, London SW12 9PU, UK 3Current address: Zoological Society of London, Regents Park, London NW1 4RY, UK 4FORCERT, Walindi Nature Centre, Talasea Highway, West New Britain Province, Papua New Guinea 5Papua New Guinea Forest Research Institute, PO Box 314, Lae, Morobe Province, Papua New Guinea *Correspondence: Jeff Dawson < [email protected]> Abstract From June, 2007, to February, 2009, the Waria Valley Community Conservation and Sustainable Livelihoods Project (WVCP) completed an inventory survey of the birds of the lower Waria Valley, Morobe Province, Papua New Guinea. Four land use types -- agricultural, secondary forest edge, primary forest edge and primary forest -- were surveyed using Mackinnon list surveys. In total, 125 species representing 43 families were identified, of which 54 (43.2%) are endemic to the islands of New Guinea and the Bismark Archipelago. The avifauna of primary forest edge and primary forest was more species rich and diverse than that of agricultural habitats. Agricultural habitats also differed significantly in both overall community composition and some aspects of guild composition compared to all three forested habitats. Nectarivores and insectivore-frugivores formed a significantly larger proportion of species in agricultural habitats, whereas obligate frugivores formed a significantly greater proportion in forested habitats. -
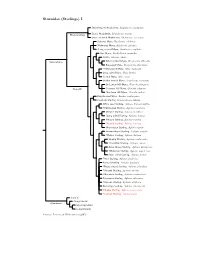
Sturnidae Tree, Part 1
Sturnidae (Starlings) I Stripe-headed Rhabdornis, Rhabdornis mystacalis Grand Rhabdornis, Rhabdornis grandis Rhabdornithini Stripe-breasted Rhabdornis, Rhabdornis inornatus Sulawesi Myna, Basilornis celebensis ?Helmeted Myna, Basilornis galeatus ?Long-crested Myna, Basilornis corythaix Apo Myna, Goodfellowia mirandus Coleto, Sarcops calvus Graculinae White-necked Myna, Streptocitta albicollis Bare-eyed Myna, Streptocitta albertinae ?Yellow-faced Myna, Mino dumontii Long-tailed Myna, Mino kreffti Golden Myna, Mino anais Golden-crested Myna, Ampeliceps coronatus Sri Lankan Hill-Myna, Gracula ptilogenys Graculini Common Hill Myna, Gracula religiosa ?Southern Hill Myna, Gracula indica Fiery-browed Myna, Enodes erythrophris Grosbeak Starling, Scissirostrum dubium White-eyed Starling, Aplonis brunneicapillus ?Yellow-eyed Starling, Aplonis mystacea Metallic Starling, Aplonis metallica ?Long-tailed Starling, Aplonis magna Pohnpei Starling, Aplonis pelzelni ?Kosrae Starling, Aplonis corvina Micronesian Starling, Aplonis opaca Brown-winged Starling, Aplonis grandis ?Makira Starling, Aplonis dichroa Singing Starling, Aplonis cantoroides ?Tanimbar Starling, Aplonis crassa Asian Glossy Starling, Aplonis panayensis ?Moluccan Starling, Aplonis mysolensis Short-tailed Starling, Aplonis minor ?Atoll Starling, Aplonis feadensis Rennell Starling, Aplonis insularis ?Rusty-winged Starling, Aplonis zelandica ?Striated Starling, Aplonis striata ?Mountain Starling, Aplonis santovestris Polynesian Starling, Aplonis tabuensis ?Samoan Starling, Aplonis atrifusca Rarotonga Starling, Aplonis cinerascens ?Mauke Starling, Aplonis mavornata ?Tasman Starling, Aplonis fusca Sturnini Cinnyricinclini Sturninae Onychognathini Lamprotornini Sources: Lovette and Rubenstein (2007).. -

AFA in Action... Newsandviews JUNE 1993
AFA in action... NEWSandVIEWS JUNE 1993 something other than a possible source of revenue. Careless President's Message consumptive use of other nations' wildlife will encourage strict Conservation small grants suspended; a sign of hard governmental regulations. A friend who is a native of a Third economic times or a question of priorities? World Nation recently asked" Why should Americans be able The American Federation of Aviculture has a long standing to buy and sell our countries' endangered species in an effort to history of providing "se~d money" for avian research and make a fast buck?" conservation projects. In 1982, the research fund awarded its Having exotic birds is a privilege. Recall that we are not able first five grants. Five years later, in 1987, the conservation fund to legally possess our own bird life. If the privilege is abused was established to aid in important avian field conservation on the local level, you may have to get rid of your birds. If it is studies. Over the years, the conservation fund has provided abused on a national level, we all face the possibility of having funds for the study of nearly every kind of bird imaginable to deal with endless permits and inspections. The success we from trogons to tanagers, hornbills to hawk-eagles. For years, have in breeding birds frequently will not make a major impact the only criterion was that the species needed some conserva on our own lifestyles but it may determine the very existence tion action. Recently, however, as AFA's own .. in house" of our birds on planet earth. -

Dr Peter Hunt
Australian Quality Awards Foundation You'llfind Caltex behind each one of these doors. These projects in the field of academic excellence in the stamp their mark in the world. education are some of the many National Scholarship for Women, And that's that Caltex supports for today's to the nurturing ofbusiness talents important if young Australians. in the Young Achievers, Caltex is we're to achieve From the encouragement of giving Australians the chance to our full potential. COMMITTED TO AUSTRALIA'S FUTURE LBC'M CCA 0015 Up Front Koalas and tree-kangaroos often exist in small, isolated colonies with greatly fluctuating populations. Now that their numbers are no longer curtailed by hunting, some colonies are faced with serious overpopulation problems. Unfortunately managing them isn't as simple as relocating colonies, so conservation managers face some tough decisions. Which spiders do you fear most? Odds on it's funnel-webs. Do you stomp on your shoes before :, :,;: z < inserting your foot or carefully check the bottom of �-..,, � ':- ....... � the pool in case one lurks there? These spiders are � 3 often accredited with legendary powers, so we sent < V funnel-web expert Mike Gray on a myth-breaking mission. Working directly across the corridor from him has its moments, as sometimes on particularly hot days, Mike has cooled off his heat stressed subjects by putting them in our fridge! Another summertime paranoia is the fear of sharks. These maligned creatures are detested and feared by people, yet we are more likely to be killed by a lightning strike than a shark! Indeed, people are a greater threat to sharks-for every person killed by a shark over 23 million kilograms of sharks and rays are killed by people. -

Flora and Fauna Monitoring Plan
FLORA AND FAUNA SUBCONTRACTOR’S CI MONITORING PLAN PAGE TINA RIVER HYDROPOWER HEC-CDSB-CESMP-MP-005 Rev. 5 1 OF 79 DEVELOPMENT PROJECT Flora and Fauna Monitoring Plan Person Responsible HEC HSE Manager REV DATE DESCRIPTION PREPARED CHECKED APPROVED 1 31/12/2019 Version 1 issued for Lender’s Review HEC 2 08/05/2020 Version 2 issued for OE’s Review HEC 3 02/06/2020 Version 2 issued for Lenders’ Review – HEC OE(JG) Access Road Lot 1 4 17/09/2020 Version 3 issued for Lenders’ Review – HEC OE (JG) Access Road 5 13/11/2020 Version 3 resubmitted for Lenders’ Review HEC OE (KS,JG) – Access Road FLORA AND FAUNA SUBCONTRACTOR’S CI MONITORING PLAN PAGE TINA RIVER HYDROPOWER HEC-CDSB-CESMP-MP-005 Rev. 5 2 OF 79 DEVELOPMENT PROJECT Revision Log Date Revised Detail Rev. (dd/mm/yyyy) Item Page Article Description FLORA AND FAUNA SUBCONTRACTOR’S CI MONITORING PLAN PAGE TINA RIVER HYDROPOWER HEC-CDSB-CESMP-MP-005 Rev. 5 3 OF 79 DEVELOPMENT PROJECT Table of Contents 1. INTRODUCTION ........................................................................................................... 6 1.1 Purpose and Scope .......................................................................................................... 6 1.2 Project Overview ............................................................................................................. 7 1.3 Institutional and Legal Framework .................................................................................. 8 1.3.1 Institutional Framework .............................................................................. -

Singing Starlings Aplonis Cantoroides and Other Birds on Boigu Island, Torres Strait, Queensland
AUSTRALIAN 20 BIRD WATCHER AUSTRALIAN BIRD WATCHER 1997, 17, 20-24 Singing Starlings Aplonis cantoroides and Other Birds on Boigu Island, Torres Strait, Queensland by MIKE CARTER1, RORY O'BRIEN2 and NEIL MACUMBER3 130 Canadian Bay Road, Mt Eliza, Victoria 3930 23 Valentine Avenue, Kew, Victoria 3101 3C/- Post Office, Pomonal, Victoria 3381 Summary Ornithological reports from islands in Torres Strait are scarce. Birds seen during a visit to Boigu Island in July 1995 are listed. Significant sightings include Singing Starling Aplonis cantoroides (first authenticated record for Australia), Whiskered Tern Chlidonias hybridus, fig-parrot Cyclopsitta sp., Frilled Monarch Arses telescopthalmus, Northern Fantail Rhipidura rufiventris and House Sparrow Passer domesticus. Introduction Boigu Island is situated about 10 km south of the coast of Papua New Guinea (PNG) in Torres Strait, Queensland, at 9 °17 'S, 142 °13 'E (Draffan et al. 1983). It is the most northerly part of Australia. The island of 7150 ha has one small settlement, with most of the remainder forest and fresh-water swamp (O'Brien 1995). As a consequence of its proximity to PNG, its avifauna is more New Guinean than Australian. We visited Boigu from 18 to 20 July 1995, and here provide an annotated list of 60 species recorded (Appendix 1). All observations were within 3 km of the village. The swampy nature of the terrain makes access to most of the island difficult. We added 15 species to the 72 listed for the island by Draffan et al. (1983) in just two days, indicating the lack of ornithological reports from the island. -

Climate Change and Queensland Biodiversity
Climate Change and Queensland Biodiversity An independent report commissioned by the Department of Environment and Resource Management (Qld) Tim Low © Author: Tim Low Date: March 2011 Citation: Low T. (2011) Climate Change and Terrestrial Biodiversity in Queensland. Department of Environment and Resource Management, Queensland Government, Brisbane. On the Cover: The purple-necked rock wallaby (Petrogale purpureicollis) inhabits a very rocky region – the North-West Highlands – where survival during heatwaves and droughts depends on access to shady rock shelters. Rising temperatures will render many of their smaller shade refuges unusuable. Photo: Brett Taylor Paperbarks (Melaleuca leucadendra) are the trees at most risk from sea level rise, because they are habitat dominants on recently formed plains near the sea where freshwater settles. They were probably scarce when the sea fell during glacials, and tend to support less biodiversity than older forest types. Photo: Jeanette Kemp, DERM Contents 1. Introduction and summary 1 5. Ecological framework 52 1.1 Introduction 1 5.1 The evidence base 53 1.2 Summary 4 5.1.1 Climatically incoherent distributions 53 1.3 Acknowledgements 5 5.1.2 Introduced species distributions 56 5.1.3 Experimental evidence 58 2. Climate change past and future 7 5.1.4 Genetic evidence 58 5.1.5 Fossil evidence 58 2.1 Temperature 8 5.2 Why distributions might not reflect climate 59 2.1.1 Past temperatures 9 5.2.1 Physical constraints 60 2.2 Rainfall 10 5.2.2 Fire 62 2.2.1 Past rainfall 11 5.2.3 Limited dispersal 63 2.3 Drought 12 5.2.4 Evolutionary history 65 2.3.1 Past drought 12 5.2.5 Lack of facilitation 65 2.4 Cyclones 12 5.2.6 Competition 66 2.4.1 Past cyclones 12 5.2.7 Predators and pathogens 70 2.5 Fire 13 5.3 Discussion 71 2.5.1 Past fire 13 5.3.1 High altitude species 71 2.6 Sea level rise 14 5.3.2 Other species 73 2.6.1 Past sea level rise 14 5.4 Management consequences 74 3. -

Development Application Details Approved Form (Version 1.2 Effective 7 February 2020) Made Under Section 282 of the Planning Act 2016
DA Form 1 – Development application details Approved form (version 1.2 effective 7 February 2020) made under section 282 of the Planning Act 2016. This form must be used to make a development application involving code assessment or impact assessment, except when applying for development involving only building work. For a development application involving building work only, use DA Form 2 – Building work details. For a development application involving building work associated with any other type of assessable development (i.e. material change of use, operational work or reconfiguring a lot), use this form (DA Form 1) and parts 4 to 6 of DA Form 2 – Building work details. Unless stated otherwise, all parts of this form must be completed in full and all required supporting information must accompany the development application. One or more additional pages may be attached as a schedule to this development application if there is insufficient space on the form to include all the necessary information. This form and any other form relevant to the development application must be used to make a development application relating to strategic port land and Brisbane core port land under the Transport Infrastructure Act 1994, and airport land under the Airport Assets (Restructuring and Disposal) Act 2008. For the purpose of assessing a development application relating to strategic port land and Brisbane core port land, any reference to a planning scheme is taken to mean a land use plan for the strategic port land, Brisbane port land use plan for Brisbane core port land, or a land use plan for airport land. -

Remote Papua New Guinea
The fabulous Superb Pitta showed brilliantly and was voted Bird of the Trip (Josh Bergmark) REMOTE PAPUA NEW GUINEA 7 JULY – 3 AUGUST 2018 LEADER: MARK VAN BEIRS and JOSH BERGMARK Our unique Remote Papua New Guinea itinerary offered once again a rich variety of terrific birding experiences in some of the least visited corners of this amazing country. This year, the logistics went unusually smooth, but copious amounts of rain sabotaged our birding endeavours on several occasions. Off the eastern tip of PNG, we travelled to the island of Fergusson in the D’Entrecasteaux Islands by live aboard boat getting excellent views of displaying Goldie’s Birds-of-paradise and of Curl-crested Manucode in the lowland rainforests. We also found the rarely seen Louisiade White-eye and an adorable chick Orange- footed Scrubfowl. We reached the forested highlands of the Huon Peninsula after an exciting charter flight and an awful drive and were rewarded by magnificent looks at gorgeous Wahnes’s Parotias, splendid Huon Astrapias, shy Emperor Birds-of-paradise and gaudy Pesquet’s Parrots. The much-wanted, but modestly- attired Mottled Berryhunter showed brilliantly in its montane forest habitat. The newly built Keki Lodge in the Adelbert Mountains offered truly cracking views of a smashing male Fire-maned Bowerbird, while Marbled Frogmouth and Obscure Berrypecker added to the fun. The central mountains of New Ireland produced goodies like New Ireland Boobook, the attractive Paradise Drongo and the undescribed Bismarck Flyrobin, while our boat trip on a very nice live aboard took us to the extraordinary seabird island of Tench where we 1 BirdQuest Tour Report: REMOTE PAPUA NEW GUINEA www.birdquest-tours.com admired wonderful Nicobar Pigeons, displaying Great Frigatebirds, Red-footed Boobies, endearing White Terns and the rare Atoll Starling.