A Study Investigating the Biodiversity Value of a Native Timber Plantation Landscape in Papua New Guinea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Communally Nesting Migratory Birds Create Ecological Hot-Spots in Tropical Australia
RESEARCH ARTICLE Communally Nesting Migratory Birds Create Ecological Hot-Spots in Tropical Australia Daniel J. D. Natusch1,2*, Jessica A. Lyons2, Gregory Brown1, Richard Shine1 1 School of Life and Environmental Sciences, University of Sydney, Sydney, New South Wales, Australia, 2 Resource Evaluation and Development, Bamaga, Queensland, Australia * [email protected] Abstract Large numbers of metallic starlings (Aplonis metallica) migrate annually from New Guinea to the rainforests of tropical Australia, where they nest communally in single emergent trees a11111 (up to 1,000 birds). These aggregations create dense and species-rich faunal “hot-spots”, attracting a diverse assemblage of local consumers that utilise this seasonal resource. The starlings nested primarily in poison-dart trees (Antiaris toxicaria) near the rainforest-wood- land boundary. Surveys underneath these colonies revealed that bird-derived nutrients massively increased densities of soil invertebrates and mammals (primarily wild pigs) beneath trees, year-round. Flying invertebrates, nocturnal birds, reptiles, and amphibians OPEN ACCESS congregated beneath the trees when starlings were nesting (the wet-season). Diurnal birds (primarily cockatoos and bush turkeys) aggregated beneath the trees during the dry-season Citation: Natusch DJD, Lyons JA, Brown G, Shine R (2016) Communally Nesting Migratory Birds Create to utilise residual nutrients when the starlings were not nesting. The abundance of several Ecological Hot-Spots in Tropical Australia. PLoS ONE taxa was considerably higher (to > 1000-fold) under colony trees than under nearby trees. 11(10): e0162651. doi:10.1371/journal.pone.0162651 The system strikingly resembles utilisation of bird nesting colonies by predators in other Editor: Maura (Gee) Geraldine Chapman, University parts of the world but this spectacular system has never been described, emphasizing the of Sydney, AUSTRALIA continuing need for detailed natural-history studies in tropical Australia. -

June 2019 Number 191
June 2019 Number 191 In this issue... June Excursion..................................1 9 June - World Swallowtail Day.............................................................2 Orchard Swallowtail.............2 Ulysses Swallowtail.................3 Cairns Birdwing........................3 Amorphophallus - Camouflagued or just pretty? .......................................................................4 In flower this month......................5 What's Happening.........................6 The rare Megahertzia amplexicaulis in cultivation at Bayview Heights. Photo by Anthony Lagois via Facebook. Cairns Branch.............................6 Townsville Branch....................6 Tablelands Branch...................6 June Excursion June's excursion will take us to the private garden of Anthony Lagois and Brian Moran. Situated on the Cairns hillslopes in Bayview Heights, the garden contains a unique and expanding collection of native rainforest plants. Many things grown here are rarely seen in cultivation. This month's excursion will commence a little earlier than usual - 10 a.m. See the last page for directions and parking instructions. Page 1 SGAP Cairns Branch - Newsletter 191 9 June - World Swallowtail Day The British "Swallowtail and Birdwing Butterfly Trust" have declared 9 June 2019 to be the World Swallowtail Day. This British conservation initiative provides an opportunity to discuss some of our native swallowtail butterflies, and the native plants they eat. Britain's swallowtail butterfly, Papilio machaon is the island nation's -

Papua New Guinea Ii
The Greater Bird-of-paradise display we witnessed at the km 17 lek in Kiunga was truly unforgettable. PAPUA NEW GUINEA II 12– 28 August / 1 September 2016 LEADER: DANI LOPEZ VELASCO Our second tour to Papua New Guinea – including New Britain - in 2016 was a great success and delivered an unprecedented number of high quality birds. A total of 21 species of Birds-of-paradise (BoPs), - undoubtedly one of the most extraordinary, and “out of this world” bird families in the world, were recorded, perhaps most memorable being a superb male Blue BoP, scoped at close range near Kumul for as long as we wished and showing one of the most vivid blue colours in the animal world. Just as impressive though were spectacular performances by displaying Raggiana and Greater BoPs in excellent light, with up to 8 males lekking at a time, a stunning male King BoP and two displaying males Twelve-wired BoPs at the Elevala River, a cracking adult male Magnificent BoP in the scope for hours at Tabubil, several amazing King-of-Saxony BoPs, waving their incredible head plumes like some strange insect antennae in the mossy forest of Tari Valley, great sightings of both Princess Stephanie´s and Ribbon-tailed Astrapias with their ridiculously long tail feathers, superb scope studies of Black and Brown Sicklebills uttering their machine-gun like calls, and so on. While Birds-of-paradise are certainly the signature family in PNG, there is of course 1 BirdQuest Tour Report: Papua New Guinea II www.birdquest-tours.com plenty more besides, for example we recorded a grand total of 33 species of pigeons and doves, -they reach their greatest diversity here in New Guinea, as do kingfishers-, including nine Fruit Doves, a rare New Guinea Bronzewing feeding on the road, and, during the extension, both Black Imperial Pigeon and Pied Cuckoo-Dove. -

Ultimate Papua New Guinea Ii
The fantastic Forest Bittern showed memorably well at Varirata during this tour! (JM) ULTIMATE PAPUA NEW GUINEA II 25 AUGUST – 11 / 15 SEPTEMBER 2019 LEADER: JULIEN MAZENAUER Our second Ultimate Papua New Guinea tour in 2019, including New Britain, was an immense success and provided us with fantastic sightings throughout. A total of 19 Birds-of-paradise (BoPs), one of the most striking and extraordinairy bird families in the world, were seen. The most amazing one must have been the male Blue BoP, admired through the scope near Kumul lodge. A few females were seen previously at Rondon Ridge, but this male was just too much. Several males King-of-Saxony BoP – seen displaying – ranked high in our most memorable moments of the tour, especially walk-away views of a male obtained at Rondon Ridge. Along the Ketu River, we were able to observe the full display and mating of another cosmis species, Twelve-wired BoP. Despite the closing of Ambua, we obtained good views of a calling male Black Sicklebill, sighted along a new road close to Tabubil. Brown Sicklebill males were seen even better and for as long as we wanted, uttering their machine-gun like calls through the forest. The adult male Stephanie’s Astrapia at Rondon Ridge will never be forgotten, showing his incredible glossy green head colours. At Kumul, Ribbon-tailed Astrapia, one of the most striking BoP, amazed us down to a few meters thanks to a feeder especially created for birdwatchers. Additionally, great views of the small and incredible King BoP delighted us near Kiunga, as well as males Magnificent BoPs below Kumul. -

WVCP Bird Paper
Mongabay.com Open Access Journal - Tropical Conservation Science Vol.4 (3):317-348, 2011 Research Article Bird communities of the lower Waria Valley, Morobe Province, Papua New Guinea: a comparison between habitat types 1* 2,3 1,4 Jeff Dawson , Craig Turner , Oscar Pileng , Andrew Farmer1, Cara McGary1, Chris Walsh1, Alexia Tamblyn2 and Cossey Yosi5 1Coral Cay Conservation, 1st Floor Block, 1 Elizabeth House, 39 York Road, London SW1 7NQ UK 2Previous address: Jaquelin Fisher Associates, 4 Yukon Road, London SW12 9PU, UK 3Current address: Zoological Society of London, Regents Park, London NW1 4RY, UK 4FORCERT, Walindi Nature Centre, Talasea Highway, West New Britain Province, Papua New Guinea 5Papua New Guinea Forest Research Institute, PO Box 314, Lae, Morobe Province, Papua New Guinea *Correspondence: Jeff Dawson < [email protected]> Abstract From June, 2007, to February, 2009, the Waria Valley Community Conservation and Sustainable Livelihoods Project (WVCP) completed an inventory survey of the birds of the lower Waria Valley, Morobe Province, Papua New Guinea. Four land use types -- agricultural, secondary forest edge, primary forest edge and primary forest -- were surveyed using Mackinnon list surveys. In total, 125 species representing 43 families were identified, of which 54 (43.2%) are endemic to the islands of New Guinea and the Bismark Archipelago. The avifauna of primary forest edge and primary forest was more species rich and diverse than that of agricultural habitats. Agricultural habitats also differed significantly in both overall community composition and some aspects of guild composition compared to all three forested habitats. Nectarivores and insectivore-frugivores formed a significantly larger proportion of species in agricultural habitats, whereas obligate frugivores formed a significantly greater proportion in forested habitats. -
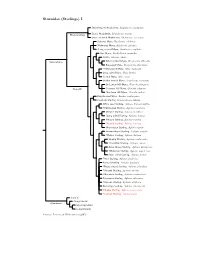
Sturnidae Tree, Part 1
Sturnidae (Starlings) I Stripe-headed Rhabdornis, Rhabdornis mystacalis Grand Rhabdornis, Rhabdornis grandis Rhabdornithini Stripe-breasted Rhabdornis, Rhabdornis inornatus Sulawesi Myna, Basilornis celebensis ?Helmeted Myna, Basilornis galeatus ?Long-crested Myna, Basilornis corythaix Apo Myna, Goodfellowia mirandus Coleto, Sarcops calvus Graculinae White-necked Myna, Streptocitta albicollis Bare-eyed Myna, Streptocitta albertinae ?Yellow-faced Myna, Mino dumontii Long-tailed Myna, Mino kreffti Golden Myna, Mino anais Golden-crested Myna, Ampeliceps coronatus Sri Lankan Hill-Myna, Gracula ptilogenys Graculini Common Hill Myna, Gracula religiosa ?Southern Hill Myna, Gracula indica Fiery-browed Myna, Enodes erythrophris Grosbeak Starling, Scissirostrum dubium White-eyed Starling, Aplonis brunneicapillus ?Yellow-eyed Starling, Aplonis mystacea Metallic Starling, Aplonis metallica ?Long-tailed Starling, Aplonis magna Pohnpei Starling, Aplonis pelzelni ?Kosrae Starling, Aplonis corvina Micronesian Starling, Aplonis opaca Brown-winged Starling, Aplonis grandis ?Makira Starling, Aplonis dichroa Singing Starling, Aplonis cantoroides ?Tanimbar Starling, Aplonis crassa Asian Glossy Starling, Aplonis panayensis ?Moluccan Starling, Aplonis mysolensis Short-tailed Starling, Aplonis minor ?Atoll Starling, Aplonis feadensis Rennell Starling, Aplonis insularis ?Rusty-winged Starling, Aplonis zelandica ?Striated Starling, Aplonis striata ?Mountain Starling, Aplonis santovestris Polynesian Starling, Aplonis tabuensis ?Samoan Starling, Aplonis atrifusca Rarotonga Starling, Aplonis cinerascens ?Mauke Starling, Aplonis mavornata ?Tasman Starling, Aplonis fusca Sturnini Cinnyricinclini Sturninae Onychognathini Lamprotornini Sources: Lovette and Rubenstein (2007).. -

Birding Melanesia 2015 Report by Adam Walleyn
Melanesia Discover and Secrets of Melanesia: Birding Melanesia 2015 Report By Adam Walleyn Cardinal Lory pair. Copyright Adrian Hayward The 2015 Melanesian Birding trip was another great success. The year will probably long be remembered for one of the worst droughts ever and while the dry and windy conditions made birding more difficult than usual, we persevered and ended up with an incredible tally of endemics, many of them amongst the most poorly known birds in the world! This incredible itinerary takes in part of the north coast of Papua New Guinea and all of the main islands of the Bismarcks, Solomons and Vanuatu, along with many of the smaller ones. This region is one of the world’s most avian endemic-rich hotspots and is largely inaccessible and unvisited by birders. Amongst 267 species, highlights this year included Superb Pitta sitting right in the open, an unexpected Manus Fantail, one of the first observations of Mussau Triller, a stunning Solomons Nightjar, and incredible diversity of fruit doves (12 species), imperial pigeons (12 species), myzomelas (11 species) and of course white-eyes (10 species). The trip started off with a nice dinner in Madang and then our first of many early mornings to bird a patch of forest not far from town. Bird activity was great this morning and there were a number of fruiting trees which allowed good views of two species of birds of paradise - Lesser Bird of Paradise and Glossy-mantled Manucode. Other nice birds in the fruiting trees included Orange-bellied and Pink-spotted Fruit Dove, Zoe’s Imperial Pigeon, Orange-breasted Fig Parrot, and numerous Golden Myna. -

TELOPEA Publication Date: 26 September 1990 Til
Volume 4(1): 33–35 TELOPEA Publication Date: 26 September 1990 Til. Ro)'al BOTANIC GARDENS dx.doi.org/10.7751/telopea19904915 Journal of Plant Systematics 6 DOPII(liPi Tmst plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL· ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) 33 A new species and new combinations in Melicope (Rutaceae) in New South Wales T.G. Hartley Abstract Hartley, T.G. (Australian National Herbarium, Division of Plant Industry, CSIRO, G.P.O. Box 1600, Canberra, A.CT. 2601) 1990. A new species and new combinations in Melicope (Rutaceae) in New South Wales. Telopea 4(1): 33-35. Melicope hayesii T. Hartley is described as new and new combi nations in Me/icope are made for three species previously included in Euodia. Melicope J.R. & G. Forster and Euodia J.R. & G. Forster are being revised. The notes that follow are provided so that the names are available for use in the forthcoming Flora of New South Wales. The new classification of Melicope and Euodia has been discussed by Hartley (1981) and Hartley and Stone (1989). Melicope hayesii T. Hartley, sp. nov. Arbor 5-18 malta; ramulis et foliis glabris; foliis trifoliolatis, 6.5-16 cm longis; petiolo, 2-6 cm longo; lamina foliolorum lateralium ovato-elliptica, anguste ovato-elliptica, elliptica, vel anguste elliptica lamina terminali elliptica vel anguste elliptica, 3.7-9 x 1.2-4 cm; inflorescentiis axillaribus, multifloris, 2.5-6 x 2.5-5 cm, axe et ramis glabris vel fere glabris, pedicellis glabris vel sparse et minute puberulis, 2-4.5 mm longis; floribus bisexualibus; petalis albis, abaxialiter glabris, adaxialiter in 1/2 proximali villosis, 3-3.5 mm longis, caducis; staminibus 4; gynoecio glabro; carpellis fructificantibus in 1/5 proximali connatis, divergentibus, c. -

West New Britain Extension July 22–27, 2017
WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 White-mantled Kingfisher (Dion Hobcroft) LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 By Dion Hobcroft A Purple-bellied Lory dines out in a Coconut flower. (Dion Hobcroft) Our tour got off to a shaky start with a cancelled flight from Port Moresby to Hoskins. Luckily, the superb Airways Hotel honored our rooms from the previous day that we had missed due to another cancelled flight. Love you Air Niugini! After settling in to the very comfortable Walindi Dive Resort, with its well-planned rooms, tasty meals, and excellent staff, we headed out for our first birding in New Britain. We met Joel, a local villager, who guided us up beyond his village into the forest. He led us to a New Britain Boobook, a small hawk-owl he keeps tabs on (and has done so for the past few years). With the elections on, I joked with Joel that we should call the owl “Prime Minister Pete.” We had actually seen the Prime Minister the day before. Victor Emanuel Nature Tours 2 West New Britain Extension, 2017 After great looks at “Pete” we slowly wandered to a viewing area over the forest that was heaving with birds —lots of Eclectus Parrots, our first Blue-eyed Cockatoos, dozens of Red-knobbed and Yellowish imperial-pigeons, Long-tailed Myna, Variable Goshawk, an all-white Pied Coucal, a single Channel-billed Cuckoo (quite rare on NB), the beautiful Purple-bellied Lory, the scarce Black-bellied Myzomela, and great views of the colorful Knob-billed Fruit-Dove. -

New Plant Records for the Hawaiian Islands 2010–20111
Records of the Hawaii Biological Survey for 2011. Edited by 27 Neal L. Evenhuis & Lucius G. Eldredge. Bishop Museum Occasional Papers 113: 27 –54 (2012) New plant records for the Hawaiian Islands 2010 –2011 1 DANielle FRoHliCH 2 & A lex lAU 2 O‘ahu Early Detection, Bishop Museum, 1525 Bernice Street, Honolulu, Hawai‘i 96817-2704; emails: [email protected]; [email protected] o‘ahu early Detection here documents 26 new naturalized records, 8 new state records, 31 new island records, 1 range extension, and 2 corrections found by us and other indi - viduals and agencies. in addition, several species showing signs of naturalization are men - tioned. A total of 42 plant families are discussed. information regarding the formerly known distribution of flowering plants is based on the Manual of the flowering plants of Hawai‘i (Wagner et al . 1999) and information subse - quently published in the Records of the Hawai ‘i Biological Survey . Voucher specimens are deposited at Bishop Museum’s Herbarium Pacificum (BiSH), Honolulu, Hawai‘i. Acanthaceae Megaskepasma erythroclamys lindau New island record This species, which was previously found naturalizing on o‘ahu, can be distinguished by its 1 –2" long showy burgundy bracts and white, tubular, 2-lipped corollas with 2 fertile stamens (Staples & Herbst 2005). Parker & Parsons (this volume) report this species as naturalized on Hawai‘i island. Material examined . KAUA ‘I: Hā‘ena, in neighborhood makai of highway, near Tunnels Beach, UTM 442390, 2457621. Coastal residential setting; sparingly-branched shrub to 6 ft tall, growing out of a hedge. inflorescence bracts magenta. Species is planted as an ornamental and sparingly natural - ized in the area, 9 Mar 2010, OED 2010030904. -

Papua New Guinea New Britain Extension III 30Th August to 3Rd September 2019 (5 Days)
Papua New Guinea New Britain Extension III 30th August to 3rd September 2019 (5 days) Trip Report Blue-eyed Cockatoo by Chris Lester Trip report compiled by Dušan Brinkhuizen & Stephan Lorenz Trip Report – RBL Papua New Guinea – New Britain III 2019 2 Top ten birds as voted for by participants: 1. Golden Masked Owl 6. Melanesian Megapode 2. New Britain Boobook 7. Bismarck Pitta 3. Black-capped Paradise Kingfisher 8. Violaceous Coucal 4. Pied Cuckoo-Dove 9. Nicobar Pigeon 5. Black Imperial Pigeon 10. Purple-bellied Lory __________________________________________________________________________________ Tour Summary New Britain is the largest island in the Bismarck Archipelago and located just to the northeast of mainland Papua New Guinea. We had three full days to explore this beautiful but little-known island in search of the region’s best endemics. We arrived at Hoskins airport in the late afternoon and made it to our comfortable resort for check-in just around dusk. Apart from Bismarck Crow, Willy Wagtail and Blyth's Hornbill, not many other species were identified during our drive to Kimbe Bay. The following morning, we started our first proper birding at Garu Wildlife Management Area, one of the few protected areas in New Britain. At arrival there was some good activity of parrots with the fantastic Purple-bellied Lory being the first endemic to be scoped. The endemic Blue-eyed Cockatoo soon followed giving us some excellent flight views. Eclectus Parrots and Coconut Lorikeets were also flying around and the tiny Buff-faced Pygmy Parrot was scoped in the canopy. Suddenly, Stephan called out three flyover pigeons which turned out to be the rare Black Imperial Pigeon – what a great catch! Walking down the forest track, we quickly stacked up several other regional endemics such as Red-banded Flowerpecker, White-necked Coucal, Long-tailed Myna and New Britain Friarbird, all of them showing well. -

Report on the Vegetation of the Proposed Blue Hole Cultural, Environmental & Recreation Reserve
Vegetation Report on the Proposed Blue Hole Cultural, Environmental & Recreation Reserve Report on the Vegetation of the Proposed Blue Hole Cultural, Environmental & Recreation Reserve 1.0 Introduction The area covered by this report is described as the proposed Lot 1 on SP144713; Parish of Alexandra; being an unregistered plan prepared by the C & B Group for the Douglas Shire Council. This proposed Lot has an area of 1.394 hectares and consists of the Flame Tree Road Reserve and part of a USL, which is a small portion of the bed of Cooper Creek. It is proposed that the Flame Tree Road Reserve and part of the USL be transferred to enable the creation of a Cultural, Environmental and Recreation Reserve to be managed in Trust by the Douglas Shire Council. The proposed Cultural, Environmental and Recreation Reserve will have an area of 1.394 hectares and will if the plan is registered become Lot 1 of SP144713; Parish of Alexandra; County of Solander. It is proposed that three Easements A, B & C over the proposed Lot 1 of SP144713 be created in favour of Lot 180 RP739774, Lot 236 RP740951, Lot 52 of SR537 and Lot 51 SR767 as per the unregistered plan SP 144715 prepared by the C & B Group for the Douglas Shire Council. 2.0 Trustee Details Douglas Shire Council 64-66 Front Street Mossman PO Box 357 Mossman, Qld, 4873 Phone: (07) 4099 9444 Fax: (07) 4098 2902 Email: [email protected] Internet: www.dsc.qld.gov.au 3.0 Description of the Subject Land The “Blue Hole” is a local name for a small pool in a section of Cooper Creek.