Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Nutritional Relationships of Zinc David L
The Nutritional Relationships of Zinc David L. Watts, D.C., Ph.D., F.A.C.E.P.i Zinc was discovered to be essential for the also indicate tissue redistribution.12 The normal growth of living organisms in 1869. The range of zinc in the hair has been reported suspicion that zinc deficiency occurs in man is between 15 and 22 milligrams percent,4 the ideal relatively recent. In 1963, studies reported by being 20 milligrams Prasad and co-workers on Iranian men suffering percent. from dwarfism and hypogonadism found that nutritional zinc deficiency was a causative factor Manifestations of Zinc Deficiency Absolute or in these disorders.1 2 3 Since that time zinc has Relative gained a greater recognition for its role in human Manifestations of zinc deficiency will vary health and has stimulated extensive research. It is from one individual to another. This is true of now known that zinc is essential to over 100 almost any nutrient, and can be explained by enzymes in the body. Perhaps one of the most recognizing that two types of a deficiency state important discoveries is zinc's involvement in the can occur, either a relative deficiency, or synthesis of RNA. absolute deficiency. An absolute deficiency develops as a result of inhibited absorption Distribution accompanied by a concurrent increase in zinc The highest concentration of zinc is found in excretion or utilization. TMA patterns usually the choroid of the eye and optic nerve, followed reveal a low tissue zinc (less than 12 mg.%). An by the prostate, bone, liver and kidneys, muscles absolute deficiency of zinc can be contributed to (zinc content varies with colour and function of by hypoadrenocorticism, hyperthyroidism and muscles), heart, spleen, testes, brain, and other endocrine factors. -

The Skin in the Ehlers-Danlos Syndromes
EDS Global Learning Conference July 30-August 1, 2019 (Nashville) The Skin in the Ehlers-Danlos Syndromes Dr Nigel Burrows Consultant Dermatologist MD FRCP Department of Dermatology Addenbrooke’s Hospital Cambridge University NHS Foundation Trust Cambridge, UK No conflict of interests or disclosures Burrows, N: The Skin in EDS 1 EDS Global Learning Conference July 30-August 1, 2019 (Nashville) • Overview of skin and anatomy • Skin features in commoner EDS • Skin features in rarer EDS subtypes • Skin management The skin • Is useful organ to sustain life ØProtection - microorganisms, ultraviolet light, mechanical damage ØSensation ØAllows movement ØEndocrine - vitamin D production ØExcretion - sweat ØTemperature regulation Burrows, N: The Skin in EDS 2 EDS Global Learning Conference July 30-August 1, 2019 (Nashville) The skin • Is useful organ to sustain life • Provides a visual clue to diagnoses • Important for cultures and traditions • Ready material for research Skin Fun Facts • Largest organ in the body • In an average adult the skin weighs approx 5kg (11lbs) and covers 2m2 (21 sq ft) • 11 miles of blood vessels • The average person has about 300 million skin cells • More than half of the dust in your home is actually dead skin • Your skin is home to more than 1,000 species of bacteria Burrows, N: The Skin in EDS 3 EDS Global Learning Conference July 30-August 1, 2019 (Nashville) The skin has 3 main layers Within the Dermis Extracellular Matrix 1. Collagen 2. Elastic fibres 3. Ground Substances i) glycosaminoglycans, ii) proteoglycans, -

Rapid Development of Perifolliculitis Following Mesotherapy
CASE LETTER Rapid Development of Perifolliculitis Following Mesotherapy Weihuang Vivian Ning, MD; Sameer Bashey, MD; Gene H. Kim, MD patient received mesotherapy with an unknown substance PRACTICE POINTS for cosmetic rejuvenation; the rash was localized only to the injection sites.copy She did not note any fever, chills, • Mesotherapy—the delivery of vitamins, chemicals, and plant extracts directly into the dermis via nausea, vomiting, diarrhea, headache, arthralgia, or upper injections—is a common procedure performed respiratory tract symptoms. She further denied starting in both medical and nonmedical settings for any new medications, herbal products, or topical therapies cosmetic rejuvenation. apart from the procedure she had received 2 weeks prior. • Complications can occur from mesotherapy treatment. Thenot patient was found to be in no acute distress and • Patients should be advised to seek medical care with vital signs were stable. Laboratory testing was remarkable US Food and Drug Administration–approved cosmetic for elevations in alanine aminotransferase (62 U/L [refer- techniques and substances only. ence range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had Doan absolute neutrophil count of 0.5×103 cells/µL (refer- ence range 1.8–8.0×103 cells/µL). An electrolyte panel, To the Editor: creatinine level, and urinalysis were normal. Physical Mesotherapy, also known as intradermotherapy, is a examination revealed numerous 4- to 5-mm erythematous cosmetic procedure in which multiple -

Inflammatory Or Infectious Hair Disease? a Case of Scalp Eschar and Neck Lymph Adenopathy After a Tick Bite
Case Report ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2021.35.005688 Adherent Serous Crust of the Scalp: Inflammatory or Infectious Hair Disease? A Case of Scalp Eschar and Neck Lymph Adenopathy after a Tick Bite Starace M1, Vezzoni R*2, Alessandrini A1 and Piraccini BM1 1Dermatology - IRCCS, Policlinico Sant’Orsola, Department of Specialized, Experimental and Diagnostic Medicine, Alma Mater Studiorum, University of Bologna, Italy 2Dermatology Clinic, Maggiore Hospital, University of Trieste, Italy *Corresponding author: Roberta Vezzoni, Dermatology Clinic, Maggiore Hospital, University of Trieste, Italy ARTICLE INFO ABSTRACT Received: Published: April 17, 2021 The appearance of a crust initially suggests inflammatory scalp diseases, although infectious diseases such as impetigo or insect bites should also be considered among April 27, 2021 the differential diagnoses. We report a case of 40-year-old woman presentedB. Burgdorferi to our, Citation: Starace M, Vezzoni R, Hair Disease Outpatient Service with an adherent serous crust on the scalp and lymphadenopathy of the neck. Serological tests confirmed the aetiology of while rickettsia infection was excluded. Lyme borreliosis can mimic rickettsia infection Alessandrini A, Piraccini BM. Adherent and may present as scalp eschar and neck lymphadenopathy after a tick bite (SENLAT). Serous Crust of the Scalp: Inflammatory Appropriate tests should be included in the diagnostic workup of patients with necrotic or Infectious Hair Disease? A Case of Scalp scalpKeywords: eschar in order to promptly set -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

Interstitial Granuloma Annulare Triggered by Lyme Disease
Volume 27 Number 5| May 2021 Dermatology Online Journal || Case Presentation 27(5):11 Interstitial granuloma annulare triggered by Lyme disease Jordan Hyde1 MD, Jose A Plaza1,2 MD, Jessica Kaffenberger1 MD Affiliations: 1Division of Dermatology, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA, 2Department of Pathology, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA Corresponding Author: Jessica Kaffenberger MD, Division of Dermatology, The Ohio State University Medical Wexner Medical Center, Suite 240, 540 Officenter Place, Columbus, OH 43230, Tel: 614-293-1707, Email: [email protected] been associated with a variety of systemic diseases Abstract including diabetes mellitus, malignancy, thyroid Granuloma annulare is a non-infectious disease, dyslipidemia, and infection [3,4]. granulomatous skin condition with multiple different associations. We present a case of a man in his 60s There are multiple histological variants of GA, with a three-week history of progressive targetoid including interstitial GA. The histopathology of plaques on his arms, legs, and trunk. Skin biopsy classic GA demonstrates a focal degeneration of demonstrated interstitial granuloma annulare. collagen surrounded by an inflammatory infiltrate Additional testing revealed IgM antibodies to Borrelia composed of lymphocytes and histiocytes. In a less burgdorferi on western blot suggesting interstitial common variant, interstitial GA, scattered histiocytes granuloma annulare was precipitated by the recent are seen -

Bartonella Henselae • Fleas and Black-Legged Ticks (Also Called Deer Ticks) of the Genus Ixodes May Serve As Vectors, but This Has Not Disease Agent: Been Proven
APPENDIX 2 Bartonella henselae • Fleas and black-legged ticks (also called deer ticks) of the genus Ixodes may serve as vectors, but this has not Disease Agent: been proven. • Bartonella henselae Blood Phase: Disease Agent Characteristics: • Agent found in endothelial cells and associated with RBCs in symptomatic cases • Gram-negative bacillus or coccobacillus, aerobic, • Occult bacteremia sometimes occurs in the absence nonmotile, nonspore-forming, facultatively intracel- of specific antibodies. lular bacterium • Order: Rhizobiales; Family: Bartonellaceae Survival/Persistence in Blood Products: • Size: 0.3-0.6 ¥ 0.3-1.0 mm • Nucleic acid: Approximately 1900 kb of DNA • A spiking study suggests that B. henselae added to RBCs can be recovered on solid media through 35 Disease Name: days of storage at 4°C. • Cat scratch disease • Cat scratch fever Transmission by Blood Transfusion: • Bacillary angiomatosis • Theoretical • Bacillary peliosis Cases/Frequency in Population: Priority Level: • 22,000 cases per year estimated in the US • Scientific/Epidemiologic evidence regarding blood • 2-6% in US blood donors safety: Theoretical • Cumulative seroprevalence of 7.1% to B. henselae and • Public perception and/or regulatory concern regard- B. quintana in US veterinary professionals ing blood safety: Absent • Public concern regarding disease agent: Very low Incubation Period: Background: • 3-10 days to appearance of papule at inoculation site; regional adenopathy may follow after a few weeks • In 1909,ALBartondescribed organisms that adhered to RBCs. Likelihood of Clinical Disease: • The name Bartonella bacilliformis was used for the • Relatively benign and self-limiting, lasting 6-12 weeks only member of the group identified before 1993. in the absence of antibiotic therapy • Several other species of Bartonella are known to infect humans, but at present, B. -

Urticaria and Prodromal Symptoms Including Erythema Marginatum in Danish Patients with Hereditary Angioedema
University of Southern Denmark Urticaria and Prodromal Symptoms Including Erythema Marginatum in Danish Patients with Hereditary Angioedema Rasmussen, Eva R; Valente de Freitas, Priscila; Bygum, Anette Published in: Acta Dermatovenereologica DOI: 10.2340/00015555-2233 Publication date: 2016 Document version: Final published version Document license: CC BY Citation for pulished version (APA): Rasmussen, E. R., Valente de Freitas, P., & Bygum, A. (2016). Urticaria and Prodromal Symptoms Including Erythema Marginatum in Danish Patients with Hereditary Angioedema. Acta Dermatovenereologica, 96(3), 373- 376. https://doi.org/10.2340/00015555-2233 Go to publication entry in University of Southern Denmark's Research Portal Terms of use This work is brought to you by the University of Southern Denmark. Unless otherwise specified it has been shared according to the terms for self-archiving. If no other license is stated, these terms apply: • You may download this work for personal use only. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying this open access version If you believe that this document breaches copyright please contact us providing details and we will investigate your claim. Please direct all enquiries to [email protected] Download date: 07. Oct. 2021 Acta Derm Venereol 2016; 96: 373–376 CLINICAL REPORT Urticaria and Prodromal Symptoms Including Erythema Marginatum in Danish Patients with Hereditary Angioedema Eva Rye RASMUSSEN1, -

Erythema Marginatum
Figurative Erythemas Michelle Goedken, DO Affiliated Dermatology Scottsdale, AZ Figurative Erythemas • Erythema annulare centrifugum • Erythema marginatum • Erythema migrans • Erythema gyratum repens • Erythema multiforme Erythemas • Erythemas represent a change in the color of the skin that is due to the dilation of blood vessels, especially those in the papillary and reticular dermis • The color is blanchable and most last for days to months • Figurative erythemas have an annular, arciform or polycyclic appearance ERYTHEMA ANNULARE CENTRIFUGUM ERYTHEMA ANNULARE CENTRIFUGUM • Pathogenesis: EAC represents a reaction pattern or hypersensitivity to one of many antigens – IL-2 and TNF-alpha may have a role – Most patients do not have an underlying disease identified ERYTHEMA ANNULARE CENTRIFUGUM • Associated with: – Infection » Dermatophytes and other fungi (Candida and Penicillium in blue cheese) » Viruses: poxvirus, EBV, VZV, HIV » Parasites and ectoparasites – Drugs: diuretics, antimalarials, gold, NSAIDs, finasteride, amitriptyline, etizolam, Ustekinumab (2012) ERYTHEMA ANNULARE CENTRIFUGUM – Foods – Autoimmune endocrinopathies – Neoplasms (lymphomas and leukemias) – Pregnancy – Hypereosinophilic syndrome – Lupus (2014) ERYTHEMA ANNULARE CENTRIFUGUM http://www.dermaamin.com Rongioletti, F., Fausti, V., & Parodi, A ERYTHEMA ANNULARE CENTRIFUGUM • 2 major forms: – Superficial: classic trailing scale, may have associated pruritus – Deep: infiltrated borders, usually no scale, edges are elevated, usually not pruritic ERYTHEMA ANNULARE CENTRIFUGUM -

Bartonella Henselae Detected in Malignant Melanoma, a Preliminary Study
pathogens Article Bartonella henselae Detected in Malignant Melanoma, a Preliminary Study Marna E. Ericson 1, Edward B. Breitschwerdt 2 , Paul Reicherter 3, Cole Maxwell 4, Ricardo G. Maggi 2, Richard G. Melvin 5 , Azar H. Maluki 4,6 , Julie M. Bradley 2, Jennifer C. Miller 7, Glenn E. Simmons, Jr. 5 , Jamie Dencklau 4, Keaton Joppru 5, Jack Peterson 4, Will Bae 4, Janet Scanlon 4 and Lynne T. Bemis 5,* 1 T Lab Inc., 910 Clopper Road, Suite 220S, Gaithersburg, MD 20878, USA; [email protected] 2 Intracellular Pathogens Research Laboratory, Comparative Medicine Institute, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA; [email protected] (E.B.B.); [email protected] (R.G.M.); [email protected] (J.M.B.) 3 Dermatology Clinic, Truman Medical Center, University of Missouri, Kansas City, MO 64108, USA; [email protected] 4 Department of Dermatology, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] (C.M.); [email protected] (A.H.M.); [email protected] (J.D.); [email protected] (J.P.); [email protected] (W.B.); [email protected] (J.S.) 5 Department of Biomedical Sciences, Duluth Campus, Medical School, University of Minnesota, Duluth, MN 55812, USA; [email protected] (R.G.M.); [email protected] (G.E.S.J.); [email protected] (K.J.) 6 Department of Dermatology, College of Medicine, University of Kufa, Kufa 54003, Iraq 7 Galaxy Diagnostics Inc., Research Triangle Park, NC 27709, USA; [email protected] Citation: Ericson, M.E.; * Correspondence: [email protected]; Tel.: +1-720-560-0278; Fax: +1-218-726-7906 Breitschwerdt, E.B.; Reicherter, P.; Maxwell, C.; Maggi, R.G.; Melvin, Abstract: Bartonella bacilliformis (B. -
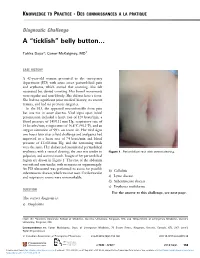
Belly Button…
KNOWLEDGE TO PRACTICE DES CONNAISSANCES À LA PRATIQUE Diagnostic Challenge A “ticklish” belly button… Tahira Daya*; Conor McKaigney, MD† CASE HISTORY A 42-year-old woman presented to the emergency department (ED) with acute onset periumbilical pain and erythema, which started that morning. She felt nauseated but denied vomiting. Her bowel movements were regular and non-bloody. She did not have a fever. She had no significant prior medical history, no recent trauma, and had no previous surgeries. In the ED, she appeared uncomfortable from pain but was not in acute distress. Vital signs upon initial presentation included a heart rate of 120 beats/min, a blood pressure of 145/111 mm Hg, respiratory rate of 16 breaths/min, temperature of 36.8°C (98.2°F), and an oxygen saturation of 99% on room air. Her vital signs two hours later after a fluid challenge and analgesics had improved to a heart rate of 74 beats/min and blood pressure of 132/83 mm Hg, and the remaining vitals were the same. Her abdomen demonstrated periumbilical erythema, with a central clearing; the area was tender to Figure 1. Periumbilical rash with central clearing. palpation, and warm to touch. Images of her periumbilical region are shown in Figure 1. The rest of the abdomen was soft and non-tender, with no masses or organomegaly. An ED ultrasound was performed to assess for possible b) Cellulitis subcutaneous abscess, which was not seen. Cardiovascular and respiratory exams were unremarkable. c) Lyme disease d) Subcutaneous abscess e) Erythema multiforme QUESTION For the answer to this challenge, see next page. -
Bacteria Clostridia Bacilli Eukaryota CFB Group
AM935842.1.1361 uncultured Burkholderiales bacterium Class Betaproteobacteria AY283260.1.1552 Alcaligenes sp. PCNB−2 Class Betaproteobacteria AM934953.1.1374 uncultured Burkholderiales bacterium Class Betaproteobacteria AJ581593.1.1460 uncultured betaAM936569.1.1351 proteobacterium uncultured Class Betaproteobacteria Derxia sp. Class Betaproteobacteria AJ581621.1.1418 uncultured beta proteobacterium Class Betaproteobacteria DQ248272.1.1498 uncultured soil bacterium soil uncultured DQ248272.1.1498 DQ248235.1.1498 uncultured soil bacterium RS49 DQ248270.1.1496 uncultured soil bacterium DQ256489.1.1211 Variovorax paradoxus Class Betaproteobacteria Class paradoxus Variovorax DQ256489.1.1211 AF523053.1.1486 uncultured Comamonadaceae bacterium Class Betaproteobacteria AY706442.1.1396 uncultured bacterium uncultured AY706442.1.1396 AJ536763.1.1422 uncultured bacterium CS000359.1.1530 Variovorax paradoxus Class Betaproteobacteria Class paradoxus Variovorax CS000359.1.1530 AY168733.1.1411 uncultured bacterium AJ009470.1.1526 uncultured bacterium SJA−62 Class Betaproteobacteria Class SJA−62 bacterium uncultured AJ009470.1.1526 AY212561.1.1433 uncultured bacterium D16212.1.1457 Rhodoferax fermentans Class Betaproteobacteria Class fermentans Rhodoferax D16212.1.1457 AY957894.1.1546 uncultured bacterium AJ581620.1.1452 uncultured beta proteobacterium Class Betaproteobacteria RS76 AY625146.1.1498 uncultured bacterium RS65 DQ316832.1.1269 uncultured beta proteobacterium Class Betaproteobacteria DQ404909.1.1513 uncultured bacterium uncultured DQ404909.1.1513 AB021341.1.1466 bacterium rM6 AJ487020.1.1500 uncultured bacterium uncultured AJ487020.1.1500 RS7 RS86RC AF364862.1.1425 bacterium BA128 Class Betaproteobacteria AY957931.1.1529 uncultured bacterium uncultured AY957931.1.1529 CP000884.723807.725332 Delftia acidovorans SPH−1 Class Betaproteobacteria AY957923.1.1520 uncultured bacterium uncultured AY957923.1.1520 RS18 AY957918.1.1527 uncultured bacterium uncultured AY957918.1.1527 AY945883.1.1500 uncultured bacterium AF526940.1.1489 uncultured Ralstonia sp.