A SIMPLIFIED METHOD for IDENTIFYING the PREDOMINANT CLAY MINERAL in SOIL by Eugene C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nature of Interlayer Material in Silicate Clays of Selected Oregon Soils
AN ABSTRACT OF THE THESIS OF PAUL C, SINGLETON for the Ph.D. in Soils (Name) (Degree) (Major) Date thesis is presented July 28, 1965 Title NATURE OF INTERLAYER MATERIAL IN SILICATE CLAYS OF SELECTED OREGON SOILS - Redacted for Privacy Abstract approved = ajor professor) Ç A study was conducted to investigate the nature of hydroxy interlayers in the chlorite -like intergrade clays of three Oregon soils with respect to kind, amount, stability, and conditions of formation. The clays of the Hembre, Wren, and Lookout soils, selected to represent weathering products originating from basaltic materials under humid, subhumid, and semi -arid climatic conditions respectively, were subjected to a series of progressive treatments designed to effect a differential dissolution of the materials intimately asso- ciated with them. The treatments, chosen to represent a range of increasing severity of dissolution, were (1) distilled water plus mechanical stirring, (2) boiling 2% sodium carbonate, (3) buffered sodium citrate -dithionite, (4) boiling sodium hydroxide, and (5) preheating to 400 °C for 4 hours plus boiling sodium hydroxide. Extracts from the various steps of the dissolution procedure were chemically analyzed in order to identify the materials removed from the clays. X -ray diffraction analysis and cation exchange capacity determinations were made on the clays after each step, and any differences noted in the measured values were attributed to the removal of hydroxy interlayers from the clays. Hydroxy interlayers were found to occur more in the Hembre and Wren soils than in the Lookout soil, with the most stable interlayers occurring in the Wren. Soil reaction was one of the major differences between these soils. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Bio-Preservation Potential of Sediment in Eberswalde Crater, Mars
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Fall 2020 Bio-preservation Potential of Sediment in Eberswalde crater, Mars Cory Hughes Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Hughes, Cory, "Bio-preservation Potential of Sediment in Eberswalde crater, Mars" (2020). WWU Graduate School Collection. 992. https://cedar.wwu.edu/wwuet/992 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. Bio-preservation Potential of Sediment in Eberswalde crater, Mars By Cory M. Hughes Accepted in Partial Completion of the Requirements for the Degree Master of Science ADVISORY COMMITTEE Dr. Melissa Rice, Chair Dr. Charles Barnhart Dr. Brady Foreman Dr. Allison Pfeiffer GRADUATE SCHOOL David L. Patrick, Dean Master’s Thesis In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files. -

An Investigation Into Transitions in Clay Mineral Chemistry on Mars
UNLV Theses, Dissertations, Professional Papers, and Capstones 8-31-2015 An Investigation into Transitions in Clay Mineral Chemistry on Mars Seth Gainey University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Geochemistry Commons, Geology Commons, and the Mineral Physics Commons Repository Citation Gainey, Seth, "An Investigation into Transitions in Clay Mineral Chemistry on Mars" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2475. http://dx.doi.org/10.34917/7777303 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. AN INVESTIGATION INTO TRANSITIONS IN CLAY MINERAL CHEMISTRY ON MARS By Seth R. Gainey Bachelor of Science in Geology St. Cloud State University 2009 Master of Science in Geology University of Oklahoma 2011 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy – Geoscience Department of Geoscience College of Sciences The Graduate College University of Nevada, Las Vegas August 2015 Copyright by Seth R. -

Clay Mineralogy Techniques
ITATI OP OHIO DIPAlTMINT OP NATURAL lUOUlCIS DIVISION OF &EOLO&ICAL SURVEY INFORMATION CIRCULAR NO. 20 CLAY MINERALOGY TECHNIQUES - A REVIEW- by Merril I F. Au kla nd COLUMBUS 1956 SECOND PRINTING 1959 STA TE OF omo Michael V. DiSalle Governor DEPARTMENT OF NATURAL RESOURCES Herbert B. Eagon Director NATURAL RESOURCES COMMISSION C. D. Blubaugh L. L. Rummell Herbert B. Eagon Demas L. Sears Byron Frederick James R. Stephenson Forrest G. Hall Myron T. Sturgeon William Hoyne DIVISION OF GEOLOGICAL SURVEY Ralph J. Bernhagen Chief r STATI OF OHIO ! I DIPAlTMINT 011 NATURAL lUOUICH I DIVISION OF GEOLOGICAL SURVEY INFORMATION CIRCULAR NO. 20 CLAY MINERALOGY TECHNIQUES - A REVIEW- by Merrill F. Aukland COLUMBUS 1956 SECOND PRINTING 1959 Blank Page CONTENTS Page INTRODUCTION 1 DEFINITIONS . 1 CLASSIFICATION AND NOMENCLATURE OF CLAY AND CLAY MINERALS .............. 2 PHYSICAL PROPERTIES OF CLAY MATERIALS 5 General . ...... 5 Particle size and composition. 7 Bonding strength . 8 Firing properties . 9 Differential thermal analysis . 10 X-ray diffraction . 12 Optical properties . 13 Summary of physical properties. 14 ORIGIN AND OCCURRENCE OF CLAY AND CLAY MINERALS 16 STRUCTURAL MINERALOGY OF CLAYS 18 CONCLUSIONS. 22 BIBLIOGRAPHY . 23 APPENDIX ... 27 ILLUSTRATIONS Figure 1. Miscellaneous differential thermal curves 11 2. Structure of kaolinite (Grim) 19 3. Diagram of the crystal structure of kaolinite (after Gruner) . 20 4. Diagram indicating the crystal structure of pyrophyllite (after Pauling). 20 iii INTRODUCTION The study of clays and the clay minerals is of considerable magnitude when it is realized that it is a subject pertaining to several closely integrated sciences and applied sciences. Among the sciences are chemistry, physics, mineralogy, and geology; and in the field of applied science, ceramics, engineering, and agriculture. -

Crystallochemical Characterization of the Palygorskite and Sepiolite from the Allou Kagne Deposit, Senegal
CRYSTALLOCHEMICAL CHARACTERIZATION OF THE PALYGORSKITE AND SEPIOLITE FROM THE ALLOU KAGNE DEPOSIT, SENEGAL 2 E. GARCÍA-RoMER01,*, M. SUÁREZ , J. SANTARÉN3 AND A. ALVAREZ3 1 Departamento de Cristalografía y Mineralogía, Universidad Complutense de Madrid, E-28040 Madrid, Spain 2 Departamento de Geología, Universidad de Salamanca, E-37008 Salamanca, Spain 3 TOLSA Ctra Vallecas-Mejorada del Campo, km 1600, 28031 Madrid, Spain Abstract-The Allou Kagne (Senegal) deposit consists of different proportions of palygorskite and sepiolite, and these are associated with small quantities of quartz and X-ray amorphous silica as impurities. No pure palygorskite or sepiolite has been recognized by X-ray diffraction. Textural and microtextural features indicate that fibrous clay minerals ofthe Allou Kagne deposit were formed by direct precipitation from solution. Crystal-chemistry data obtained by analyticalltransmission electron microscopy (AEMI TEM) analyses of isolated fibers show that the chemical composition of the particles varies over a wide range, from a composition corresponding to palygorskite to a composition intermediate between that of sepiolite and palygorskite, but particles with a composition corresponding to sepiolite have not been found. Taking mto account the results from selected area electron diffraction and AEM-TEM, fibers of pure palygorskite and sepiolite have been found but it cannot be confirmed that all of the particles analyzed correspond to pure palygorskite or pure sepiolite because both minerals can occur together at the crystallite scale. In addition, the presence ofMg-rich palygorskite and very Al-rich sepiolite can be deduced. It is infrequent in nature that palygorskite and sepiolite appear together because the conditions for simultaneous formation ofthe two minerals are very restricted. -

Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications
minerals Review Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications Marina Fomina * and Iryna Skorochod Zabolotny Institute of Microbiology and Virology of National Academy of Sciences of Ukraine, Zabolotny str., 154, 03143 Kyiv, Ukraine; [email protected] * Correspondence: [email protected] Received: 13 August 2020; Accepted: 24 September 2020; Published: 29 September 2020 Abstract: Clay minerals are very common in nature and highly reactive minerals which are typical products of the weathering of the most abundant silicate minerals on the planet. Over recent decades there has been growing appreciation that the prime involvement of clay minerals in the geochemical cycling of elements and pedosphere genesis should take into account the biogeochemical activity of microorganisms. Microbial intimate interaction with clay minerals, that has taken place on Earth’s surface in a geological time-scale, represents a complex co-evolving system which is challenging to comprehend because of fragmented information and requires coordinated efforts from both clay scientists and microbiologists. This review covers some important aspects of the interactions of clay minerals with microorganisms at the different levels of complexity, starting from organic molecules, individual and aggregated microbial cells, fungal and bacterial symbioses with photosynthetic organisms, pedosphere, up to environmental and biotechnological implications. The review attempts to systematize our current general understanding of the processes of biogeochemical transformation of clay minerals by microorganisms. This paper also highlights some microbiological and biotechnological perspectives of the practical application of clay minerals–microbes interactions not only in microbial bioremediation and biodegradation of pollutants but also in areas related to agronomy and human and animal health. -

Clay Mineral Distribution in Surface Sediments of the South Atlantic: Sources, Transport, and Relation to Oceanography
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Electronic Publication Information Center Manuskript für Marine Geology Clay Mineral Distribution in Surface Sediments of the South Atlantic: Sources, Transport, and Relation to Oceanography Rainer Petschick1) 2), Gerhard Kuhn1), and Franz Gingele1) with 13 Figures, 1 Table 1) Alfred-Wegener-Institut für Polar- und Meeresforschung, Columbusstr., 27515 Bremerhaven, Germany 2) Present Adress: Geologisch-Paläontologisches Institut, J.W.Goethe University, Senckenberganlage 32-34, 60054 Franfurt am Main, Germany 2 Abstract 850 surface samples mostly from abyssal sediments of the South Atlantic and the Antarctic Ocean were investigated for clay content and composition. Maps of relative clay mineral content were compiled, which improve previous maps by showing more details, especially at high latitudes. Large-scaled relations regarding the origin and transport paths of detrital clay are revealed. Near submarine volcanoes of the Antarctic Ocean (South Sandwich, Bouvet Island) smectite contents exhibit distinct maxima, which is ascribed to the erosion of altered basalts and volcanic glasses. Other areas of high smectite concentration are observed in abyssal regions, primarily derived from southernmost America and from minor sources in Southwest Africa. The illite distribution can be subdivided into five major zones including two maxima revealing both South African and Antarctican sources. A particulary high amount of Fe- and Mg-rich illites are observed close to East Antarctica derived from biotite bearing crystalline rocks and transported to the west by the East Antarctic Coastal current. Chlorites and well-crystallized illites are typical minerals enriched within the Subantarctic and Polarfrontal-Zone but of minor importance off East Antarctica. -
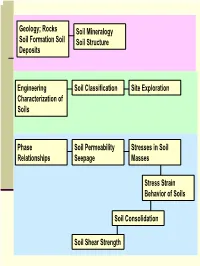
Rocks Soil Formation Soil Deposits Soil Mineralogy Soil Structure
Geology; Rocks Soil Mineralogy Soil Formation Soil Soil Structure Deposits Engineering Soil Classification Site Exploration Characterization of Soils Phase Soil Permeability Stresses in Soil Relationships Seepage Masses Stress Strain Behavior of Soils Soil Consolidation Soil Shear Strength 1 Engineering Characterization of Soils Soil Properties that Control its Engineering Behavior Particle Size − Sieve Analysis − Hydrometer Analysis coarse-grained fine-grained Particle/Grain Size Soil Plasticity Distribution Particle Shapes (?) 2 Particle Size; Standard Sieve Sizes 3 ASTM Particle Size Classification 4 Sieve Analysis (Mechanical Analysis) This procedure is suitable for coarse grained soils See next slide for ASTM Standard Sieves No.10 sieve …. Has 10 apertures per linear inch 5 ASTM Standard Sieves 6 Hydrometer Analysis Also called Sedimentation Analysis Stoke’s Law D2γ (G − G ) v = w s L 18η 7 Grain Size Distribution Curves 8 Terminology C….. Poorly-graded soil D …. Well-graded soil E …. Gap-graded soil D10, D30, D60 = ?? Coefficient of Uniformity, Cu= D60/D10 Coefficient of Curvature, )2 Cc= (D30 /(D10)(D60) 9 Particle Distribution Calculations Example 10 Particle Shapes 11 Clay Formation Clay particles < 2 µm Compared to Sands and Silts, clay size particles have undergone a lot more “chemical weathering”! 12 Clay vs. Sand/Silt Clay particles are generally more platy in shape (sand more equi-dimensional) Clay particles carry surface charge Amount of surface charge depends on type of clay minerals Surface charges that -

Clay Minerals
CLAY MINERALS CD. Barton United States Department of Agriculture Forest Service, Aiken, South Carolina, U.S.A. A.D. Karathanasis University of Kentucky, Lexington, Kentucky, U.S.A. INTRODUCTION of soil minerals is understandable. Notwithstanding, the prevalence of silicon and oxygen in the phyllosilicate structure is logical. The SiC>4 tetrahedron is the foundation Clay minerals refers to a group of hydrous aluminosili- 2 of all silicate structures. It consists of four O ~~ ions at the cates that predominate the clay-sized (<2 |xm) fraction of apices of a regular tetrahedron coordinated to one Si4+ at soils. These minerals are similar in chemical and structural the center (Fig. 1). An interlocking array of these composition to the primary minerals that originate from tetrahedral connected at three corners in the same plane the Earth's crust; however, transformations in the by shared oxygen anions forms a hexagonal network geometric arrangement of atoms and ions within their called the tetrahedral sheet (2). When external ions bond to structures occur due to weathering. Primary minerals form the tetrahedral sheet they are coordinated to one hydroxyl at elevated temperatures and pressures, and are usually and two oxygen anion groups. An aluminum, magnesium, derived from igneous or metamorphic rocks. Inside the or iron ion typically serves as the coordinating cation and Earth these minerals are relatively stable, but transform- is surrounded by six oxygen atoms or hydroxyl groups ations may occur once exposed to the ambient conditions resulting in an eight-sided building block termed an of the Earth's surface. Although some of the most resistant octohedron (Fig. -

Dispersion Characteristics of Montmorillonite, Kaolinite, and Hike Clays in Waters of Varying Quality, and Their Control with Phosphate Dispersants by B
Dispersion Characteristics of Montmorillonite, Kaolinite, and Hike Clays in Waters of Varying Quality, and Their Control with Phosphate Dispersants By B. N. ROLFE, R. F. MILLER, and I. S. McQUEEN SHORTER CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 334-G Prepared in cooperation with Colorado State University UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1960 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. - Price 40 cents (paper cover) CONTENTS Page Results and discussion Continued Page Abstract._ ________________________________________ 229 Sodium illite in hard water_______________________ 241 Introduction.______________________________________ 229 Calcium illite in hard water._____________________ 243 Personnel._____________________________________ 230 Volclay in distilled water._______________________ 243 Acknowledgments _______________________________ 230 Volclay in medium-hard water- _-_-_-_---_.___ 243 Materials----------------------_---__---_----_--__- 231 Kaolinite (78.8 percent sodium saturated) in distilled Clay minerals._________________________________ 231 water ___-_-____--_-__-__-------_-_---_--____ 246 Phosphate deflocculents_--_-_______-_-__--______ 231 Kaolinite (78.8 percent sodium saturated) in medium- Waters.--____-----_--_--___-_-__--_-________ 231 hard water...________________________________ 246 Methods_______________________._________ -

4. Grain-Size Distribution and Significance of Clay and Clay-Sized Minerals in Eocene to Holocene Sediments from Sites 918 and 919 in the Irminger Basin1
Saunders, A.D., Larsen, H.C., and Wise, S.W., Jr. (Eds.), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 152 4. GRAIN-SIZE DISTRIBUTION AND SIGNIFICANCE OF CLAY AND CLAY-SIZED MINERALS IN EOCENE TO HOLOCENE SEDIMENTS FROM SITES 918 AND 919 IN THE IRMINGER BASIN1 Kraig A. Heiden2,3 and Mary Anne Holmes2 ABSTRACT Lower Eocene to Holocene sediments recovered from Ocean Drilling Program Sites 918 and 919 were studied to determine the grain-size distribution (sand to clay sizes) and mineralogy of the <2 µm size fraction. The minerals are believed to be of detrital origin. The clay minerals consist of chlorite, smectite, illite, kaolinite, and a mixed-layer illite/smectite. Several non- clay minerals were identified as well, including quartz, plagioclase, alkali-feldspar, amphibole, pyroxene, zeolite, and calcite. Relative abundances of the clay minerals were semiquantified using an oriented internal standard. Smectite abundances were found to increase with depth, while illite and chlorite abundances decrease with depth. The Eocene sediments of Site 918 are characterized by a predominance of smectite with some kaolinite and very small amounts of chlorite and illite. This mineral assemblage is indicative of warm climatic conditions at the time of deposition. Oli- gocene sediments show an increase in chlorite and illite, suggesting that a sediment dam may have existed on the continental shelf, trapping these sediments and preventing their transport into the Irminger Basin, prior to this time. A warming trend in the early to middle Miocene is indicated by increased amounts of kaolinite. Variations in the relative amounts of chlorite and illite at this time may be the result of short-term eustatic sea level changes.