Water in the Small Bodies of the Solar System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Exoplanet Populations with NASA's Kepler Mission
SPECIAL FEATURE: PERSPECTIVE PERSPECTIVE SPECIAL FEATURE: Exploring exoplanet populations with NASA’s Kepler Mission Natalie M. Batalha1 National Aeronautics and Space Administration Ames Research Center, Moffett Field, 94035 CA Edited by Adam S. Burrows, Princeton University, Princeton, NJ, and accepted by the Editorial Board June 3, 2014 (received for review January 15, 2014) The Kepler Mission is exploring the diversity of planets and planetary systems. Its legacy will be a catalog of discoveries sufficient for computing planet occurrence rates as a function of size, orbital period, star type, and insolation flux.The mission has made significant progress toward achieving that goal. Over 3,500 transiting exoplanets have been identified from the analysis of the first 3 y of data, 100 planets of which are in the habitable zone. The catalog has a high reliability rate (85–90% averaged over the period/radius plane), which is improving as follow-up observations continue. Dynamical (e.g., velocimetry and transit timing) and statistical methods have confirmed and characterized hundreds of planets over a large range of sizes and compositions for both single- and multiple-star systems. Population studies suggest that planets abound in our galaxy and that small planets are particularly frequent. Here, I report on the progress Kepler has made measuring the prevalence of exoplanets orbiting within one astronomical unit of their host stars in support of the National Aeronautics and Space Admin- istration’s long-term goal of finding habitable environments beyond the solar system. planet detection | transit photometry Searching for evidence of life beyond Earth is the Sun would produce an 84-ppm signal Translating Kepler’s discovery catalog into one of the primary goals of science agencies lasting ∼13 h. -

Wastewater Technology Fact Sheet: Ammonia Stripping
United States Office of Water EPA 832-F-00-019 Environmental Protection Washington, D.C. September 2000 Agency Wastewater Technology Fact Sheet Ammonia Stripping DESCRIPTION Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it (Culp et al., 1978). Ammonia (a weak base) reacts with water (a weak acid) to form ammonium hydroxide. In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units which converts ammonium hydroxide ions to ammonia gas according to the following reaction(s): + - NH4 + OH 6 H2O + NH38 Source: Culp, et. al, 1978. Figure 1 illustrates two variations of ammonia FIGURE 1 TWO TYPES OF STRIPPING stripping towers, cross-flow and countercurrent. In TOWERS a cross-flow tower, the solvent gas (air) enters along the entire depth of fill and flows through the packing, as the alkaline wastewater flows it may be more economical to use alternate downward. A countercurrent tower draws air ammonia removal techniques, such as steam through openings at the bottom, as wastewater is stripping or biological methods. Air stripping may pumped to the top of a packed tower. Free also be used to remove many hydrophobic organic ammonia (NH3) is stripped from falling water molecules (Nutrient Control, 1983). droplets into the air stream, then discharged to the atmosphere. -

Ozonedisinfection.Pdf
ETI - Environmental Technology Initiative Project funded by the U.S. Environmental Protection Agency under Assistance Agreement No. CX824652 What is disinfection? Human exposure to wastewater discharged into the environment has increased in the last 15 to 20 years with the rise in population and the greater demand for water resources for recreation and other purposes. Disinfection of wastewater is done to prevent infectious diseases from being spread and to ensure that water is safe for human contact and the environment. There is no perfect disinfectant. However, there are certain characteristics to look for when choosing the most suitable disinfectant: • Ability to penetrate and destroy infectious agents under normal operating conditions; • Lack of characteristics that could be harmful to people and the environment; • Safe and easy handling, shipping, and storage; • Absence of toxic residuals, such as cancer-causing compounds, after disinfection; and • Affordable capital and operation and maintenance (O&M) costs. What is ozone disinfection? One common method of disinfecting wastewater is ozonation (also known as ozone disinfection). Ozone is an unstable gas that can destroy bacteria and viruses. It is formed when oxygen molecules (O2) collide with oxygen atoms to produce ozone (O3). Ozone is generated by an electrical discharge through dry air or pure oxygen and is generated onsite because it decomposes to elemental oxygen in a short amount of time. After generation, ozone is fed into a down-flow contact chamber containing the wastewater to be disinfected. From the bottom of the contact chamber, ozone is diffused into fine bubbles that mix with the downward flowing wastewater. See Figure 1 on page 2 for a schematic of the ozonation process. -

Formation of TRAPPIST-1
EPSC Abstracts Vol. 11, EPSC2017-265, 2017 European Planetary Science Congress 2017 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2017 Formation of TRAPPIST-1 C.W Ormel, B. Liu and D. Schoonenberg University of Amsterdam, The Netherlands ([email protected]) Abstract start to drift by aerodynamical drag. However, this growth+drift occurs in an inside-out fashion, which We present a model for the formation of the recently- does not result in strong particle pileups needed to discovered TRAPPIST-1 planetary system. In our sce- trigger planetesimal formation by, e.g., the streaming nario planets form in the interior regions, by accre- instability [3]. (a) We propose that the H2O iceline (r 0.1 au for TRAPPIST-1) is the place where tion of mm to cm-size particles (pebbles) that drifted ice ≈ the local solids-to-gas ratio can reach 1, either by from the outer disk. This scenario has several ad- ∼ vantages: it connects to the observation that disks are condensation of the vapor [9] or by pileup of ice-free made up of pebbles, it is efficient, it explains why the (silicate) grains [2, 8]. Under these conditions plan- TRAPPIST-1 planets are Earth mass, and it provides etary embryos can form. (b) Due to type I migration, ∼ a rationale for the system’s architecture. embryos cross the iceline and enter the ice-free region. (c) There, silicate pebbles are smaller because of col- lisional fragmentation. Nevertheless, pebble accretion 1. Introduction remains efficient and growth is fast [6]. (d) At approx- TRAPPIST-1 is an M8 main-sequence star located at a imately Earth masses embryos reach their pebble iso- distance of 12 pc. -

Water, Habitability, and Detectability Steve Desch
Water, Habitability, and Detectability Steve Desch PI, “Exoplanetary Ecosystems” NExSS team School of Earth and Space Exploration, Arizona State University with Ariel Anbar, Tessa Fisher, Steven Glaser, Hilairy Hartnett, Stephen Kane, Susanne Neuer, Cayman Unterborn, Sara Walker, Misha Zolotov Astrobiology Science Strategy NAS Committee, Beckmann Center, Irvine, CA (remotely), January 17, 2018 How to look for life on (Earth-like) exoplanets: find oxygen in their atmospheres How Earth-like must an exoplanet be for this to work? Seager et al. (2013) How to look for life on (Earth-like) exoplanets: find oxygen in their atmospheres Oxygen on Earth overwhelmingly produced by photosynthesizing life, which taps Sun’s energy and yields large disequilibrium signature. Caveats: Earth had life for billions of years without O2 in its atmosphere. First photosynthesis to evolve on Earth was anoxygenic. Many ‘false positives’ recognized because O2 has abiotic sources, esp. photolysis (Luger & Barnes 2014; Harman et al. 2015; Meadows 2017). These caveats seem like exceptions to the ‘rule’ that ‘oxygen = life’. How non-Earth-like can an exoplanet be (especially with respect to water content) before oxygen is no longer a biosignature? Part 1: How much water can terrestrial planets form with? Part 2: Are Aqua Planets or Water Worlds habitable? Can we detect life on them? Part 3: How should we look for life on exoplanets? Part 1: How much water can terrestrial planets form with? Theory says: up to hundreds of oceans’ worth of water Trappist-1 system suggests hundreds of oceans, especially around M stars Many (most?) planets may be Aqua Planets or Water Worlds How much water can terrestrial planets form with? Earth- “snow line” Standard Sun distance models of distance accretion suggest abundant water. -

CAPTURE of TRANS-NEPTUNIAN PLANETESIMALS in the MAIN ASTEROID BELT David Vokrouhlický1, William F
The Astronomical Journal, 152:39 (20pp), 2016 August doi:10.3847/0004-6256/152/2/39 © 2016. The American Astronomical Society. All rights reserved. CAPTURE OF TRANS-NEPTUNIAN PLANETESIMALS IN THE MAIN ASTEROID BELT David Vokrouhlický1, William F. Bottke2, and David Nesvorný2 1 Institute of Astronomy, Charles University, V Holešovičkách 2, CZ–18000 Prague 8, Czech Republic; [email protected] 2 Department of Space Studies, Southwest Research Institute, 1050 Walnut Street, Suite 300, Boulder, CO 80302; [email protected], [email protected] Received 2016 February 9; accepted 2016 April 21; published 2016 July 26 ABSTRACT The orbital evolution of the giant planets after nebular gas was eliminated from the Solar System but before the planets reached their final configuration was driven by interactions with a vast sea of leftover planetesimals. Several variants of planetary migration with this kind of system architecture have been proposed. Here, we focus on a highly successful case, which assumes that there were once five planets in the outer Solar System in a stable configuration: Jupiter, Saturn, Uranus, Neptune, and a Neptune-like body. Beyond these planets existed a primordial disk containing thousands of Pluto-sized bodies, ∼50 million D > 100 km bodies, and a multitude of smaller bodies. This system eventually went through a dynamical instability that scattered the planetesimals and allowed the planets to encounter one another. The extra Neptune-like body was ejected via a Jupiter encounter, but not before it helped to populate stable niches with disk planetesimals across the Solar System. Here, we investigate how interactions between the fifth giant planet, Jupiter, and disk planetesimals helped to capture disk planetesimals into both the asteroid belt and first-order mean-motion resonances with Jupiter. -
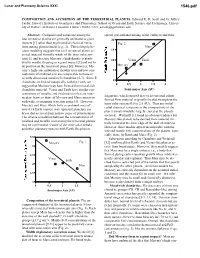
Composition and Accretion of the Terrestrial Planets
Lunar and Planetary Science XXXI 1546.pdf COMPOSITION AND ACCRETION OF THE TERRESTRIAL PLANETS. Edward R. D. Scott and G. Jeffrey Taylor, Hawai’i Institute of Geophysics and Planetology, School of Ocean and Earth Science and Technology, Univer- sity of Hawai’i at Manoa, Honolulu, Hawai’i 96822, USA; [email protected] Abstract: Compositional variations among the spread gravitational mixing of the embryos and their four terrestrial planets are generally attributed to giant 20 impacts [1] rather than to primordial chemical varia- Fig. 2 tions among planetesimals [e.g., 2]. This is largely be- Mars cause modeling suggests that each terrestrial planet ac- 15 creted material from the whole of the inner solar sys- tem [1], and because Mercury’s high density is attrib- 10 Venus Earth uted to mantle stripping in a giant impact [3] and not to its position as the innermost planet [4]. However, Mer- Mercury cury’s high concentration of metallic iron and low con- 5 centration of oxidized iron are comparable to those in recently discovered metal-rich chondrites [5-7]. Since E 0 chondrites are linked isotopically with the Earth, we 0 0.5 1 1.5 2 suggest that Mercury may have formed from metal-rich chondritic material. Venus and Earth have similar con- Semi-major Axis (AU) centrations of metallic and oxidized iron that are inter- fragments, which ensured that each terrestrial planet mediate between those of Mercury and Mars consistent formed from material originally located throughout the with wide, overlapping accretion zones [1]. However, inner solar system (0.5 to 2.5 AU). -

POSSIBLE STRUCTURE MODELS for the TRANSITING SUPER-EARTHS:KEPLER-10B and 11B
43rd Lunar and Planetary Science Conference (2012) 1290.pdf POSSIBLE STRUCTURE MODELS FOR THE TRANSITING SUPER-EARTHS:KEPLER-10b AND 11b. P. Futó1 1 Department of Physical Geography, University of West Hungary, Szombathely, Károlyi Gáspár tér, H- 9700, Hungary ([email protected]) Introduction:Up to january of 2012,10 super- The planet Kepler-11b has a large radius (1.97 R⊕) for Earths have been announced by Kepler-mission [1] its mass (4.3 M⊕),therefore this planet must have a that is designed to detect hundreds of transiting exo- spherical shell that is composed of low-density materi- planets.Kepler was launched on 6th March,2009 and the als.Considering the planet's average density,it must primary purpose of its scientific program is to search have a metallic core with different possible fractional for terrestrial-sized planets in the habitable zone of mass.Accordinghly,I have made a possible structure Solar-like stars.For the case of high number of discov- model for Kepler-11b in which the selected core mass eries we will be able to estimate the frequency of fraction is 32.59% (similarly to that of Earth) and the Earth-sized planets in our galaxy.Results of the Kepler- water ice layer has a relatively great fractional 's measurements show that the small-sized planets are volume.For case of the selected composition, the icy frequent in the spiral galaxies.A catalog of planetary surface sublimated to form a water vapor as the planet candidates,including objects with small-sized candidate moved inward the central star during its migration. -

Lecture 3 - Minimum Mass Model of Solar Nebula
Lecture 3 - Minimum mass model of solar nebula o Topics to be covered: o Composition and condensation o Surface density profile o Minimum mass of solar nebula PY4A01 Solar System Science Minimum Mass Solar Nebula (MMSN) o MMSN is not a nebula, but a protoplanetary disc. Protoplanetary disk Nebula o Gives minimum mass of solid material to build the 8 planets. PY4A01 Solar System Science Minimum mass of the solar nebula o Can make approximation of minimum amount of solar nebula material that must have been present to form planets. Know: 1. Current masses, composition, location and radii of the planets. 2. Cosmic elemental abundances. 3. Condensation temperatures of material. o Given % of material that condenses, can calculate minimum mass of original nebula from which the planets formed. • Figure from Page 115 of “Physics & Chemistry of the Solar System” by Lewis o Steps 1-8: metals & rock, steps 9-13: ices PY4A01 Solar System Science Nebula composition o Assume solar/cosmic abundances: Representative Main nebular Fraction of elements Low-T material nebular mass H, He Gas 98.4 % H2, He C, N, O Volatiles (ices) 1.2 % H2O, CH4, NH3 Si, Mg, Fe Refractories 0.3 % (metals, silicates) PY4A01 Solar System Science Minimum mass for terrestrial planets o Mercury:~5.43 g cm-3 => complete condensation of Fe (~0.285% Mnebula). 0.285% Mnebula = 100 % Mmercury => Mnebula = (100/ 0.285) Mmercury = 350 Mmercury o Venus: ~5.24 g cm-3 => condensation from Fe and silicates (~0.37% Mnebula). =>(100% / 0.37% ) Mvenus = 270 Mvenus o Earth/Mars: 0.43% of material condensed at cooler temperatures. -

Cosmochemistry Cosmic Background Radia�On
6/10/13 Cosmochemistry Cosmic background radiaon Dust Anja C. Andersen Niels Bohr Instute University of Copenhagen hp://www.dark-cosmology.dk/~anja Hauser & Dwek 2001 Molecule formation on dust grains Multiwavelenght MW 1 6/10/13 Gas-phase element depleons in the Concept of dust depleon interstellar medium The depleon of an element X in the ISM is defined in terms of (a logarithm of) its reducon factor below the expected abundance relave to that of hydrogen if all of the atoms were in the gas phase, [Xgas/H] = log{N(X)/N(H)} − log(X/H) which is based on the assumpon that solar abundances (X/H)are good reference values that truly reflect the underlying total abundances. In this formula, N(X) is the column density of element X and N(H) represents the column density of hydrogen in both atomic and molecular form, i.e., N(HI) + 2N(H2). The missing atoms of element X are presumed to be locked up in solids within dust grains or large molecules that are difficult to idenfy spectroscopically, with fraconal amounts (again relave to H) given by [Xgas/H] (Xdust/H) = (X/H)(1 − 10 ). Jenkins 2009 Jenkins 2009 2 6/10/13 Jenkins 2009 Jenkins 2009 The Galacc Exncon Curve Extinction curves measure the difference in emitted and observed light. Traditionally measured by comparing two stars of the same spectral type. Galactic Extinction - empirically determined: -1 -1 <A(λ)/A(V)> = a(λ ) + b(λ )/RV (Cardelli et al. 1999) • Bump at 2175 Å (4.6 µm-1) • RV : Ratio of total to selective extinction in the V band • Mean value is RV = 3.1 (blue) • Low value: RV = 1.8 (green) (Udalski 2003) • High value: RV = 5.6-5.8 (red) (Cardelli et al. -

Deuterium – Tritium Pulse Propulsion with Hydrogen As Propellant and the Entire Space-Craft As a Gigavolt Capacitor for Ignition
Deuterium – Tritium pulse propulsion with hydrogen as propellant and the entire space-craft as a gigavolt capacitor for ignition. By F. Winterberg University of Nevada, Reno Abstract A deuterium-tritium (DT) nuclear pulse propulsion concept for fast interplanetary transport is proposed utilizing almost all the energy for thrust and without the need for a large radiator: 1. By letting the thermonuclear micro-explosion take place in the center of a liquid hydrogen sphere with the radius of the sphere large enough to slow down and absorb the neutrons of the DT fusion reaction, heating the hydrogen to a fully ionized plasma at a temperature of ~ 105 K. 2. By using the entire spacecraft as a magnetically insulated gigavolt capacitor, igniting the DT micro-explosion with an intense GeV ion beam discharging the gigavolt capacitor, possible if the space craft has the topology of a torus. 1. Introduction The idea to use the 80% of the neutron energy released in the DT fusion reaction for nuclear micro-bomb rocket propulsion, by surrounding the micro-explosion with a thick layer of liquid hydrogen heated up to 105 K thereby becoming part of the exhaust, was first proposed by the author in 1971 [1]. Unlike the Orion pusher plate concept, the fire ball of the fully ionized hydrogen plasma can here be reflected by a magnetic mirror. The 80% of the energy released into 14MeV neutrons cannot be reflected by a magnetic mirror for thermonuclear micro-bomb propulsion. This was the reason why for the Project Daedalus interstellar probe study of the British Interplanetary Society [2], the neutron poor deuterium-helium 3 (DHe3) reaction was chosen. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk,