Islet Antibody‐Negative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Respiratory Virusreport
Surveillance Symposium Report Inside! See page 2. Respiratory Virus Report spring 2009 isirv International Symposium on Immune Correlates of Protection Against Influenza: Reassessing licensing requirements for seasonal and pandemic influenza vaccines 2-5 March 2010 Miami, Florida, United States In this issue: SPECIAL isirv International Symposium on Viral BULLETIN Respiratory Disease Surveillance ............ 2 In response to the ongoing John Watson recounts highlights from the meeting in Seville. influenza A (H1N1) outbreak, isirv has posted two new resource lists on In the Loop.................................................. 5 its website. These lists will facilitate Summaries of recent key literature in viral respiratory disease. access to current guidance and background information relative to isirv news ................................................... 6 this new public health threat. Immune Correlates Symposium announcement, updates on the Transmission and Mitigation Symposium, and Options VII. isirv is committed to providing Obituary: Graeme Laver, PhD, FRS .......... 8 accurate information to its Robert Webster eulogizes Australian researcher Graeme Laver, who made membership. Visit www.isirv.org for major contributions to our current understanding of influenza ecology more information and authoritative and antigenic drift, and to the development of subunit vaccines and sources of key professional influenza antiviral drugs information on the influenza A (H1N1) outbreak. Respiratory Virus Report spring 2009 Highlights of the isirv International Symposium on Viral Respiratory Disease Surveillance by John Watson, MB BS, MSc, FRCP, FFPH The surveillance symposium was a great success. Pilar Perez viral respiratory disease Brena of the National Centre for Microbiology at the Instituto surveillance, seasonal de Salud Carlos III, welcomed delegates to the “charming, and pandemic influenza mysterious, and warm” city of Seville, Spain. -

Graeme Laver (Graeme) Ph.D
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Virology 385 (2009) vii–viii Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/yviro Obituary William Graeme Laver (Graeme) Ph.D. FRS 1929–2008 the depression and they moved to Melbourne where Graeme attended Ivanhoe Grammar School. At the age of 16 Graeme began work as a “bottle washer” and general laboratory helper at the Hall Institute and matriculated (graduated) by attending night school. He continued working at the Hall Institute and supported himself through Melbourne University graduating with a BSc. Subsequently he did an MSc in biochemistry at Melbourne University and was supported in his PhD at London University by a Commonwealth Scientific and Industrial Research Organization (CSIRO) scholarship. Throughout his entire life Graeme was an adventurer; during his student years as a mountaineer he met his wife, Judy. After completing his PhD in London he and Judy in 1955 drove a small “Standard 10” (English Standard Motor Company) across country back to Australia through Europe, Turkey, Iran, Afghanistan, Pakistan and India to Mumbai (Bombay) then by ship to Australia. They camped en route and were occasionally advised to move on due to bandits and “enjoyed” whole legs of camel, and as guests of honor the eyeballs at impromptu feasts. Upon arrival in Mumbai Graeme found a letter at the post office from Frank Fenner offering him a job at the John Curtin School of Medical Research at the Australia National University (ANU) in Canberra, Australia. -

Master Prog Centenary Symposium.Indd
Influenza 2018: Centenary of the 1918 Pandemic Sunday 24th - Tuesday 26th June 2018 Francis Crick Institute, London UK Programme Sunday 24 June 16.00 - 16.25 Welcome Welcome John Skehel and John McCauley Francis Crick Institute, London, UK Welcome Address Tedros Adhanom Ghebreyesus Director General, WHO, Geneva, Switzerland (TBC) 16.25 - 18.25 Session 1 Chair Robert Webster St. Jude Children’s Research Hospital, Memphis, TN, USA 16.25 - 17.05 Keynote Lecture THEN AND NOW Sally Davies Chief Medical Officer for England, London, UK 17.05 - 17.45 INFLUENZA PANDEMICS OF THE LAST CENTURY Robert Webster St. Jude Children’s Research Hospital, Memphis, TN, US 17.45 - 18.25 LESSONS FROM THE 1918 VIRUS Jeffrey Taubenberger NIAID, Bethesda, MD, USA 18.30 - 19.30 DRINKS RECEPTION Manby Gallery, Francis Crick Institute 1 Monday 25 June 09.00 - 10.45 Session 2 Virus Infection/Replication Chair Robert Lamb, HHMI Northwestern University, Evanston, IL, USA 09.00 - 09.15 Introduction INFLUENZA VIRUS ENTRY INTO CELLS AND ASSEMBLY: AN OVERVIEW Robert Lamb, HHMI Northwestern University, Evanston, IL, USA 09.15 - 09.45 IMAGING INFLUENZA VIRUS MEMBRANE FUSION Peter Rosenthal Francis Crick Institute, London, UK 09.45 - 10.15 CAP-SNATCHING AND HOST-VIRAL INTERACTIONS IMPACTING VIRAL RNA SYNTHESIS Robert Krug University of Texas, Austin, TX, USA 10.15 - 10.45 FLU-VISION: TOTAL IMAGING SYSTEMS FOR ANALYZING INFLUENZA VIRUS INFECTION Yoshi Kawaoka University of Wisconsin, Madison, WI, USA and University of Tokyo, Tokyo, Japan 10.45 - 11.15 TEA / COFFEE BREAK & VIEWING -

2010-2011 Annual Report
Annual Report 2010-2011 Mastery of disease through discovery | www.wehi.edu.au Contents 1 About the institute 3 Director’s and Chairman’s report 5 Discovery 8 Cancer and Haematology 10 Stem Cells and Cancer 12 Molecular Genetics of Cancer 14 Chemical Biology 16 Molecular Medicine 18 Structural Biology 20 Bioinformatics 22 Infection and Immunity 24 Immunology The Walter and Eliza Hall Institute 26 Autoimmunity and Transplantation of Medical Research 28 Cell Signalling and Cell Death 1G Royal Parade 30 Inflammation Parkville Victoria 3052 Australia Telephone: (+61 3) 9345 2555 32 Molecular Immunology Facsimile: (+61 3) 9347 0852 34 Publications WEHI Biotechnology Centre 36 Awards 4 Research Avenue 37 Translation La Trobe R&D Park Bundoora Victoria 3086 Australia Translating our research 38 Telephone: (+61 3) 9345 2200 40 Developing our research Facsimile: (+61 3) 9345 2211 42 Patents www.wehi.edu.au www.facebook.com/WEHIresearch 43 Education www.twitter.com/WEHI_research 46 2010-11 graduates ABN 12 004 251 423 47 Seminars Acknowledgements 48 Institute awards Produced by the institute’s Community Relations department 49 Engagement Managing editor: Penny Fannin Editor: Liz Williams 51 Strategic partners Writers: Liz Williams, Vanessa Solomon and Julie Tester 52 Scientific and medical community Design and production: Simon Taplin Photography: Czesia Markiewicz and Cameron Wells 54 Public engagement 57 Engagement with schools Cover image 58 Donor and bequestor engagement Art in Science finalist 2010 Vessel webs 59 Sustainability Dr Leigh Coultas, Cancer and Haematology division 60 The Board This image shows the delicate intricacy in the developing eye of a transient population of web-like blood vessels. -

2020 Annual Report
2020 Annual Report Make this cover come alive with augmented reality. Details on inside back cover. Contents The Walter and Eliza Hall Institute About WEHI 1 of Medical Research President’s report 2 Parkville campus 1G Royal Parade Director’s report 3 Parkville Victoria 3052 Australia Telephone: +61 3 9345 2555 WEHI’s new brand launched 4 Bundoora campus 4 Research Avenue Our supporters 10 La Trobe R&D Park Bundoora Victoria 3086 Australia Exceptional science and people 13 Telephone: +61 3 9345 2200 www.wehi.edu.au 2020 graduates 38 WEHIresearch Patents granted in 2020 40 WEHI_research WEHI_research WEHImovies A remarkable place 41 Walter and Eliza Hall Institute Operational overview 42 ABN 12 004 251 423 © The Walter and Eliza Hall Institute Expanding connections with our alumni 45 of Medical Research 2021 Diversity and inclusion 46 Produced by the WEHI’s Communications and Marketing department Working towards reconciliation 48 Director Organisation and governance 49 Douglas J Hilton AO BSc Mon BSc(Hons) PhD Melb FAA FTSE FAHMS WEHI Board 50 Deputy Director, Scientific Strategy WEHI organisation 52 Alan Cowman AC BSc(Hons) Griffith PhD Melb FAA FRS FASM FASP Members of WEHI 54 Chief Operating Officer WEHI supporters 56 Carolyn MacDonald BArts (Journalism) RMIT 2020 Board Subcommittees 58 Chief Financial Officer 2020 Financial Statements 59 Joel Chibert BCom Melb GradDipCA FAICD Financial statements contents 60 Company Secretary Mark Licciardo Statistical summary 94 BBus(Acc) GradDip CSP FGIA FCIS FAICD The year at a glance 98 Honorary -

Catalogue and Musik” (Degenerate Music) Opened in Documentation of Sound) Should Düsseldorf
zagreb | 2018. XVIII. SVJETSKI KONGRES SAKSOFONISTA ODRŽAVA SE POD POKROVITELJSTVOM MILANA BANDIĆA, GRADONAČELNIKA GRADA ZAGREBA | THE XVIII WSC IS HELD UNDER THE PATRONAGE OF MR MILAN BANDIĆ, MAYOR OF THE CITY OF ZAGREB gradonačelnik grada zagreba Poštovani sudionici, dragi gosti, Zagreb prvi put ugošćuje renomirani Svjetski kongres saksofonista. Domaćin ovogodišnjega 18. kongresa je naša Muzička akademija koja je u svijetu poznata po izvrsnosti svoje Zagrebačke saksofonističke škole koju je utemeljio profesor Josip Nochta. Tijekom 5 dana, na više od 400 događanja, na brojnim mjestima u gradu će se predstaviti i susresti vrhunski glazbeni profesionalci, studenti i amateri. Bit će to, uvjeren sam, praznik glazbe, prigoda za promociju različitih stilova i izričaja te za razmjenu ideja. Svojom otvorenosti za publiku Kongres će obogatiti Zagrebačko kulturno ljeto te će doprinijeti boljem pozicioniranju Zagreba i Hrvatske na svjetskoj karti kulturnog turizma. Zahvaljujem organizatorima na uspješnoj realizaciji Kongresa, osobito red. prof. art. Draganu Sremcu, umjetničkomu ravnatelju Kongresa i prodekanu za umjetnost i poslovanje Muzičke akademije Sveučilišta u Zagrebu. Svim sudionicima želim uspješno sudjelovanje i ugodan boravak u našem gradu! Gradonačelnik Grada Zagreba Milan Bandić Major of the City of Zagreb Esteemed participants, dear guests, Zagreb is hosting the renowned World Saxophone Congress for the irst time. The host of this 18th Congress is our Academy of Music, world- famous for the excellence of its Zagreb School of Saxophone, established by professor Josip Nochta. In the course of 5 days, in more than 400 events at various locations in the city, top music professionals, students and amateurs will present themselves and meet each other. I am sure that it will be a celebration of music, an opportunity to promote various styles and expressions and to exchange ideas. -

Personal Accounts of Australian Drug Discovery at the Public-Private Interface
RESEARCH FRONT CSIRO PUBLISHING Aust. J. Chem. 2021, 74, 16–27 Account https://doi.org/10.1071/CH20244 Personal Accounts of Australian Drug Discovery at the Public–Private Interface* Jonathan B. Baell Medicinal Chemistry Theme, Monash Institute of Pharmaceutical Sciences (Parkville Campus), Monash University, Parkville, Vic. 3052, Australia. Email: [email protected] The public–private interface is a vibrant and invigorating stage for drug discovery and can allow for relatively higher risk but more rewarding research. Although adequate resourcing is a perennial challenge, persistence, optimism, and flexibility will pay dividends and can allow for a thoroughly rewarding career. In this account of chronological research experiences, selected examples are used to support this contention. Manuscript received: 5 August 2020. Manuscript accepted: 8 September 2020. Published online: 6 October 2020. Introduction ment of chorea associated with Huntington’s disease.[3] With Perhaps the most fitting way to introduce what became a career in improved activity against the elucidated pharmacological target medicinal chemistry is to outline that which might be said to (VMAT2, or vesicular monoamine transporter 2), the deuterated underpin it, this being those all-important formative postgraduate eutomer deutetrabenazine (Fig. 1) was approved by the USA studies. This began in the late 1980s at the University of FDA in early 2017 for the same indication and sold by Teva Tasmania, the chemistry department of which operated at a very Pharmaceuticals as Austedo. The first deuterated drug approved high standard, with names such as Frank Larkins (physical, Head by the FDA, deutetrabenazine has improved pharmacokinetic of Department), John Bremner (organic), Elaine Browne properties as a result of rendering the methoxy groups more (organic), Alan Arnold (spectroscopy), Michael Hitchman metabolically robust. -

Pandemics, Pills, and Politics Stefan Elbe
Pandemics, Pills, and Politics Stefan Elbe Published by Johns Hopkins University Press Elbe, Stefan. Pandemics, Pills, and Politics: Governing Global Health Security. Johns Hopkins University Press, 2018. Project MUSE. doi:10.1353/book.59674. https://muse.jhu.edu/. For additional information about this book https://muse.jhu.edu/book/59674 [ Access provided at 2 Oct 2021 15:24 GMT with no institutional affiliation ] This work is licensed under a Creative Commons Attribution 4.0 International License. Pandemics, Pills, and Politics This page intentionally left blank Pandemics, Pills, and Politics Governing Global Health Security Stefan Elbe JOHNS HOPKINS UNIVERSITY PRESS BALTIMORE To the Reader: Nothing in, or referenced in, this book should be construed as offering medical advice. Although every effort has been made to ensure the factual accuracy of the contents, no liability can be accepted for any use of the information in the book. This book was brought to publication with the generous assistance of the Euro pean Research Council. © 2018 Johns Hopkins University Press This work is also available in an OA edition, which is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License: https://creativecommons.org/licenses/by-nc-nd/4.0/. All rights reserved. Published 2018. Printed in the United States of Amer i ca on acid- free paper 9 8 7 6 5 4 3 2 1 Johns Hopkins University Press 2715 North Charles Street Baltimore, Mary land 21218 - 4363 www . press . jhu . edu Library of Congress Cataloging- in- Publication Data Names: Elbe, Stefan, 1975– author. Title: Pandemics, pills, and politics : governing global health security / Stefan Elbe. -

Annual Report 2016 Isobelle Dashwood in Ballet Under the Stars, Penrith NSW
Annual Report 2016 Isobelle Dashwood in Ballet Under the Stars, Penrith NSW. Photo Kate Longley CONTENTS 6 MESSAGE FROM THE CHAIRMAN AND EXECUTIVE DIRECTOR 8 ARTISTIC DIRECTOR’S REPORT 10 BOARD OF DIRECTORS 15 2016 OVERVIEW 20 PRODUCTIONS 36 KEY PERFORMANCE INDICATORS 40 ORCHESTRA VICTORIA 47 FINANCIAL REPORT 78 FINANCIAL SUPPORTERS 86 PARTNERS 92 DIRECTORS, ARTISTS AND EMPLOYEE STATISTICS 3 Richard House's Scent of Love. Photo Daniel Boud MESSAGE FROM THE CHAIRMAN AND EXECUTIVE DIRECTOR 2016 was an exciting year for The Australian Ballet and one in which As Australia’s national company, presenting ballet across the country, the company achieved significant success both on and off the stage. we work with many venue partners. We particularly value the close working relationships with our hometown venues, in which we are the In its 55th year the company demonstrated its commitment to “caring resident ballet company: the Sydney Opera House and Arts Centre for tradition, daring to be different” and realised important, ambitious Melbourne. We also acknowledge the support of venues which goals. Our seasons featured productions ranging from an exhilarating welcomed us during 2016: Melbourne’s Palais Theatre in St Kilda, showcase, including new works from The Australian Ballet’s emerging Canberra Theatre Centre, the Adelaide Festival Centre and many more choreographic talent, to productions featuring the luminous beauty in outer metropolitan and regional locations across Australia. Our of classical repertoire and a spectacularly moving tribute to a ballet orchestra colleagues across the country – notably Orchestra Victoria, legend, Nijinsky. Our performances showcased the artistry, talent and the Australian Opera and Ballet Orchestra, the Queensland Symphony commitment of our exceptional artists and achieved success across Orchestra and the Adelaide Symphony Orchestra – are also essential our key measures including artistic vibrancy, audience response, box- performance partners. -
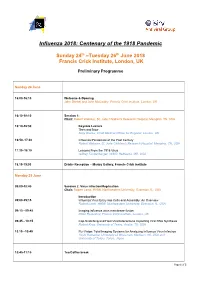
Meeting Schedule/Structure
Influenza 2018: Centenary of the 1918 Pandemic Sunday 24th –Tuesday 26th June 2018 Francis Crick Institute, London, UK Preliminary Programme Sunday 24 June 16:00-16:10 Welcome & Opening John Skehel and John McCauley, Francis Crick Institute, London, UK 16:10-18:10 Session 1: Chair: Robert Webster, St. Jude Children's Research Hospital, Memphis, TN, USA 16:10-16:50 Keynote Lecture Then and Now Sally Davies, Chief Medical Officer for England, London, UK 16:50–17.30 Influenza Pandemics of the Past Century Robert Webster, St. Jude Children's Research Hospital, Memphis, TN, USA 17.30–18.10 Lessons From the 1918 Virus Jeffrey Taubenberger, NIAID, Bethesda, MD, USA 18.15-19:30 Drinks Reception – Manby Gallery, Francis Crick Institute Monday 25 June 09:00-10:45 Session 2: Virus Infection/Replication Chair: Robert Lamb, HHMI, Northwestern University, Evanston, IL, USA Introduction 09:00-09:15 Influenza Virus Entry into Cells and Assembly: An Overview Robert Lamb, HHMI, Northwestern University, Evanston, IL, USA 09:15 - 09:45 Imaging influenza virus membrane fusion Peter Rosenthal, Francis Crick Institute, London, UK 09:45 - 10:15 Cap-Snatching and Host-Viral Interactions Impacting Viral RNA Synthesis Robert Krug, University of Texas, Austin, TX, USA 10.15 –10:45 Flu-Vision: Total Imaging Systems for Analyzing Influenza Virus Infection Yoshi Kawaoka, University of Wisconsin, Madison, WI, USA and University of Tokyo, Tokyo, Japan 10:45-11:15 Tea/Coffee break Page 1 of 3 11:15-12:30 Session 3: Antivirals Chair: Peter Colman, The Walter and Eliza -

Acknowledgment of Reviewers, 2009
Proceedings of the National Academy ofPNAS Sciences of the United States of America www.pnas.org Acknowledgment of Reviewers, 2009 The PNAS editors would like to thank all the individuals who dedicated their considerable time and expertise to the journal by serving as reviewers in 2009. Their generous contribution is deeply appreciated. A R. Alison Adcock Schahram Akbarian Paul Allen Lauren Ancel Meyers Duur Aanen Lia Addadi Brian Akerley Phillip Allen Robin Anders Lucien Aarden John Adelman Joshua Akey Fred Allendorf Jens Andersen Ruben Abagayan Zach Adelman Anna Akhmanova Robert Aller Olaf Andersen Alejandro Aballay Sarah Ades Eduard Akhunov Thorsten Allers Richard Andersen Cory Abate-Shen Stuart B. Adler Huda Akil Stefano Allesina Robert Andersen Abul Abbas Ralph Adolphs Shizuo Akira Richard Alley Adam Anderson Jonathan Abbatt Markus Aebi Gustav Akk Mark Alliegro Daniel Anderson Patrick Abbot Ueli Aebi Mikael Akke David Allison David Anderson Geoffrey Abbott Peter Aerts Armen Akopian Jeremy Allison Deborah Anderson L. Abbott Markus Affolter David Alais John Allman Gary Anderson Larry Abbott Pavel Afonine Eric Alani Laura Almasy James Anderson Akio Abe Jeffrey Agar Balbino Alarcon Osborne Almeida John Anderson Stephen Abedon Bharat Aggarwal McEwan Alastair Grac¸a Almeida-Porada Kathryn Anderson Steffen Abel John Aggleton Mikko Alava Genevieve Almouzni Mark Anderson Eugene Agichtein Christopher Albanese Emad Alnemri Richard Anderson Ted Abel Xabier Agirrezabala Birgit Alber Costica Aloman Robert P. Anderson Asa Abeliovich Ariel Agmon Tom Alber Jose´ Alonso Timothy Anderson Birgit Abler Noe¨l Agne`s Mark Albers Carlos Alonso-Alvarez Inger Andersson Robert Abraham Vladimir Agranovich Matthew Albert Suzanne Alonzo Tommy Andersson Wickliffe Abraham Anurag Agrawal Kurt Albertine Carlos Alos-Ferrer Masami Ando Charles Abrams Arun Agrawal Susan Alberts Seth Alper Tadashi Andoh Peter Abrams Rajendra Agrawal Adriana Albini Margaret Altemus Jose Andrade, Jr. -

From the Great Barrier Reef to a “Cure” for the Flu
10/Laver/Final/590–96 9/15/04 1:18 PM Page 590 From the Great Barrier Reef to a “Cure” for the Flu tall tales, but true Graeme Laver ABSTRACT How we discovered that sea birds on the Great Barrier Reef are rid- dled with influenza viruses, and how one of these led to a new drug now being used in the battle against the flu. WAS IN SYDNEY, attending a cocktail party thrown by the pharmaceutical com- I pany Hoffmann-La Roche to launch its new anti-influenza drug Tamiflu®. As one does on these occasions, I got talking to a young lady from the mar- keting division of Roche, who asked,“Why are you here? What is your involve- ment with Tamiflu?” I said,“If I told you, you wouldn’t believe me.’’ “Try me,” she replied, so this is what I said: One summer I went to a deserted coral island on Australia’s Great Barrier Reef with friends and colleagues. One of these was Adrian Gibbs, a veritable giant of a man, skilled in catching airborne objects. He caught a white-capped noddy tern, stuck a cotton wool swab up its backside, from which we isolated an influ- 3047 Barton Highway, Murrumbateman, NSW 2582,Australia. E-mail: [email protected]. Perspectives in Biology and Medicine,volume 47, number 4 (autumn 2004):590–96 © 2004 by The Johns Hopkins University Press 590 10/Laver/Final/590–96 9/15/04 1:18 PM Page 591 From the Great Barrier Reef to a “Cure” for the Flu enza virus.The neuraminidase from this virus was sent into space to crystallize on the Soviet space station Mir, and from the crystals the structure of the virus’s neuraminidase was discovered.This information was then used by Gilead Sci- ences in California to create the anti-influenza drug now called Tamiflu which was taken over by Roche and is what you are now toasting at this party.