Pollen Features in the Arum Lilies (Araceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LD5655.V855 1924.M377.Pdf
A STUDY OF BACILLUS AROIDEAE 'l'OWNSEND, THE CAUSE OF A SOFT ROT OF TOMATO, AN:) B. CAROTOVORUS JONES. A thesis 811.bmitted as partial requirement for the degree of Master of Science in Botany by A.B.Massey Reprinted from PHYT0PATH0L0GY, Vol. XIV, October, 1924. A STUDY OF BACILLUS AROIDEAE, TOWNSEND, THE CAUSE OF A SOFT ROT OF TOMATO, AND B. CAROTOVORUS JONES A. B. MASSEY! \Vl'l'll Tlll:EE FIGURES IN THE TEXT INTRODUCTION In the summer of 1918, at Blacksburg, Virginia, there developed a con- siderable amount of a soft rot of tomatoes. This occurre<l in experimental plots which were designated to study the control of septoria leaf blight, and the soft rot of the fruit developed into an important factor. I~ describing these experiments Fromme (2) states: "Practically all of the unsoundness of the fruit was caused by bacterial soft rot, a disease which is exceedingly common and often very destructive in tomato fields in Vir- ginia." Isolations from diseased fruits made by S. A. Wingard (15) proved a bacterium to be the causative agent. Its growth in pure culture resembled that of the group of bacteria which causes soft rots of plants but it could not be readily as!ligned to any of the describe<l species of this group. There has been only casual mention of a bacterial soft rot of tomato in literature, and the distinguishing features of the organisms which might be responsible have not been as sharply defined as is desirable. It was decided, therefore, to un<lcrtake comparative studies of the organism in question together with some of the non-chromogenic soft rot forms. -

Ariopsis Peltata Var. Brevifolia (Araceae) from Achankovil Shear Zone Region of Southern Western Ghats, India
Volume 18: 151–154 ELOPEA Publication date: 13 July 2015 T dx.doi.org/10.7751/telopea8757 Journal of Plant Systematics plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL • ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) Ariopsis peltata var. brevifolia (Araceae) from Achankovil Shear Zone region of Southern Western Ghats, India Jose Mathew1 and Kadasseril V. George2 1School of Environmental Sciences, Mahatma Gandhi University, Kottayam, Kerala, India. [email protected] 2NKP Vaidyar Research Foundation, Cochin, Kerala, India. [email protected] Abstract In the floristic expedition in Achankovil Shear Zone part of Southern Western Ghats, a variant of Ariopsis peltata was collected which differs from the typical species mainly by the miniature leaf size, domed male zone, and by two rings of punctured cavities in synandria. This specimen is described and illustrated here as Ariopsis peltata var. brevifolia. Introduction Achankovil Shear Zone (AKSZ) is the continuum of Mozambic belt (Pan African orogeny) of the Gondwana mass (Dissanayake and Chandrajith 1999). It extends in an area of 8 to 22 km width, which passes through the Achankovil forests of Southern Western Ghats. AKSZ is the repository of many morpho-ecotypes and endemics (Mathew and George 2013). Reasons for occurrence of morphological variants in Achankovil area have been reported due to multiple physical, climatological and geological changes that might have occurred during the evolution of the flora (Mathew 2015). During the botanical explorations in the Achankovil forests (Fig. 1) during 2009–2014 has yielded some interesting specimens of genus Ariopsis. Ariopsis peltata Nimmo is an Asiatic floral element with a distribution area extending from Southern Western Ghats to Western Malesia (Sasidharan 2013). -

Natalie Cusimano 2,11 , Josef Bogner 3 , Simon J. Mayo 4 , Peter C. Boyce 5 , Sin Y. Wong 6 , Michael Hesse 7 , Wilbert L. A. He
American Journal of Botany 98(4): 1–15. 2011. R ELATIONSHIPS WITHIN THE ARACEAE: COMPARISON OF 1 MORPHOLOGICAL PATTERNS WITH MOLECULAR PHYLOGENIES 2,11 3 4 5 Natalie Cusimano , Josef Bogner , Simon J. Mayo , Peter C. Boyce , 6 7 8 Sin Y. Wong , Michael Hesse , Wilbert L. A. Hetterscheid , Richard C. Keating 9 , and Jim C. French 10 2 Department of Biology, LMU Munich, D-80638 Munich, Germany; 3 Augsburger Str. 43a, D-86368, Gersthofen, Germany; 4 Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3AE, U.K.; 5 BRT Research Associate, Forest Herbarium (BKF), The Offi ce of Forest and Plant Conservation Research, National Park, Wildlife and Plant Conservation Department, 61 Phahonyothin Road, Chatuchak, Bangkok 10900, Thailand; 6 Department of Plant Science and Environmental Ecology, Faculty of Resource Science and Technology, Universiti Malaysia Sarawak 94300 Samarahan, Sarawak, Malaysia; 7 Department of Structural and Functional Botany, University of Vienna, 1030 Vienna, Austria; 8 Von Gimborn Arboretum, Velperengh 13, 3941 BZ Doorn, Netherlands; 9 Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, USA; and 10 188 San Jose Court, San Luis Obispo, California 93405, USA • Premise of the study: The fi rst family-wide molecular phylogeny of the Araceae, a family of about 3800 published species in 120 genera, became available in 1995, followed by a cladistic analysis of morpho-anatomical data in 1997. The most recent and comprehensive family-wide molecular phylogeny was published in 2008 and included species from 102 genera. We reanalyzed the molecular data with a more complete genus sampling and compared the resulting phylogeny with morphological and ana- tomical data, with a view to contributing to a new formal classifi cation of the Araceae. -

Leaf Variegation in Caladium Steudneriifolium (Araceae): a Case of Mimicry?
Evol Ecol (2009) 23:503–512 DOI 10.1007/s10682-008-9248-2 ORIGINAL PAPER Leaf variegation in Caladium steudneriifolium (Araceae): a case of mimicry? Ulf Soltau Æ Stefan Do¨tterl Æ Sigrid Liede-Schumann Received: 27 November 2007 / Accepted: 25 February 2008 / Published online: 6 March 2008 Ó Springer Science+Business Media B.V. 2008 Abstract The leaves of Caladium steudneriifolium (Araceae) of the understorey of a submontane rainforest in the Podocarpus National Park (South East Ecuador, 1,060 m a.s.l.) are plain green or patterned with whitish variegation. Of the 3,413 individual leaves randomly chosen and examined in April 2003, two-thirds were plain green, whereas one third were variegated (i.e., whitish due to absence of chloroplasts). Leaves of both morphs are frequently attacked by mining moth caterpillars. Our BLAST analysis based on Cytochrome-c-Oxidase-subunit-1 sequences suggests that the moth is possibly a member of the Pyraloidea or another microlepidopteran group. It was observed that the variegated leaf zones strongly resemble recent damages caused by mining larvae and therefore may mimic an attack by moth larvae. Infestation was significantly 4–12 times higher for green leaves than for variegated leaves. To test the hypothesis that variegation can be interpreted as mimicry to deter ovipositing moths, we first ruled out the possibility that variegation is a function of canopy density (i.e., that the moths might be attracted or deterred by factors unrelated to the plant). Then plain green leaves were artificially variegated and the number of mining larvae counted after 3 months. -

My Green Wet Thumb: Lagenandra
My Green Wet Thumb: Lagenandra By Derek P.S. Tustin Over the years I have found that the average aquarist will go through several different stages. I am by no means a sociologist specializing in the aquari- um hobbyist, but from my own observations I think pretty much everyone goes through some variation of the following; Initial wide-spread interest and associated errors, A focusing of interest into one or two main areas, Competence in an area of interest, Mastery of an area of interest Expansion of interest into new areas while either maintaining the old interest, or focusing entirely on the new area of interest. As an aquatic horticulturist, there are actually very few entry points, or at least entry species, into the hobby. When I started out, I had access to sev- eral excellent aquarium stores with an impressive diversity of aquatic crea- tures, but a very limited selection of aquatic plants. Now, this was back be- fore I joined the Durham Region Aquarium Society (DRAS), so I didn’t have access to mentors or their specialized stock, and it was also before there were so many excellent on-line resources. Most of my initial experience came from the limited genera of plants that were available in local stores; Echinodorus, Cryptocoryne, Anubias and some Aponogeton. (Oh, there were numerous stem plants, but for some reason, I have never been that interested in those, being much more fascinated by rooted plants, and my interest in ponds and suitable plants came much later.) Over the past decade, I have grown the majority of commonly available plants from those genera, and now also have the benefit of being exposed to other skilled hobbyists and resources offered through DRAS. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -

Caladium Genetics and Breeding: Recent Advances
® Floriculture and Ornamental Biotechnology ©2012 Global Science Books Caladium Genetics and Breeding: Recent Advances Zhanao Deng University of Florida/IFAS, Environmental Horticulture Department, Gulf Coast Research and Education Center, 14625 County Road 672, Wimauma, FL 33598, USA Corresponding author : [email protected] ABSTRACT Caladiums are important ornamental aroids; they are valued for their colourful and variably-shaped leaves. Numerous advances have been made in recent decades in caladium breeding and genetic studies. Techniques have been developed to increase flower production, store pollen, and maintain seed viability. Sources of genetic resistance have been identified for important diseases and pests (such as Fusarium tuber rot, Pythium root rot, bacterial blight, and root-knot nematodes) and abiotic stress factors including chilling injury. Mode of inheritance for important foliar traits has been elucidated through analysis of trait segregation in progeny populations. Caladiums have evolved three alleles at one locus that control colour of leaf main veins (red, white or green) and two co-dominant alleles at an independent locus that determine leaf shapes (fancy, lance, or strap). Gene loci for leaf spotting and blotching are both simply inherited but tightly linked to green veins. In vitro culture and plant regeneration were successful with several types of tissues/organs through somatic embryogenesis and/or organogenesis. Shoot-tip culture has been used to eliminate viral and fungal pathogens and invigorate planting stock; protoplasts isolated from leaf callus regenerated into whole plants; foreign genes from maize or humans have been introduced into caladium through Agrobacterium co-cultivation. Molecular markers, including highly specific and informative SSRs, have been developed and applied to caladium to distinguish cultivars, assess genetic diversity, and analyze genetic relationships. -

Is Remusatia (Araceae) Monophyletic? Evidence from Three Plastid Regions
Int. J. Mol. Sci. 2012, 13, 71-83; doi:10.3390/ijms13010071 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Is Remusatia (Araceae) Monophyletic? Evidence from Three Plastid Regions Rong Li 1,2, Tingshuang Yi 1,2 and Heng Li 1,* 1 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; E-Mails: [email protected] (R.L.); [email protected] (T.Y.) 2 Germplasm Bank of Wild Species in Southwest China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-871-5223533. Received: 18 November 2011; in revised form: 14 December 2011 / Accepted: 15 December 2011 / Published: 22 December 2011 Abstract: The genus Remusatia (Araceae) includes four species distributed in the tropical and subtropical Old World. The phylogeny of Remusatia was constructed using parsimony and Bayesian analyses of sequence data from three plastid regions (the rbcL gene, the trnL-trnF intergenic spacer, and the rps16 intron). Phylogenetic analyses of the concatenated plastid data suggested that the monophyly of Remusatia was not supported because R. hookeriana did not form a clade with the other three species R. vivipara, R. yunnanensis, and R. pumila. Nevertheless, the topology of the analysis constraining Remusatia to monophyly was congruent with the topology of the unconstrained analysis. The results confirmed the inclusion of the previously separate genus Gonatanthus within Remusatia and disagreed with the current infrageneric classification of the genus. -
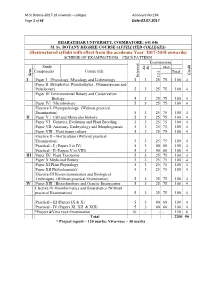
Restructured Syllabi with Effect from the Academic Year 2017-2018 Onwards
M.Sc Botany-2017-18 onwards – colleges Annexure No:19A Page 1 of 43 Date:03.07.2017 BHARATHIAR UNIVERSITY, COIMBATORE: 641 046 M. Sc. BOTANY DEGREE COURSE (AFFILIATED COLLEGES) (Restructured syllabi with effect from the academic Year 2017-2018 onwards) SCHEME OF EXAMINATIONS – CBCS PATTERN Examinations Study Mark Hrs. Dur. Components Course title Total Credit Sem. CIA Ins. hrs/week I Paper I Phycology, Mycology and Lichenology 5 3 25 75 100 4 Paper II Bryophytes, Pteridophytes , Gymnosperms and Paleobotany 5 3 25 75 100 4 Paper III Environmental Botany and Conservation Biology 5 3 25 75 100 4 Paper IV Microbiology 5 3 25 75 100 4 Elective I- Phytopathology (Without practical Examination) 5 3 25 75 100 4 II Paper V Cell and Molecular biology 5 3 25 75 100 4 Paper VI Genetics, Evolution and Plant Breeding 5 3 25 75 100 4 5 Paper VII Anatomy, Embryology and Morphogenesis 5 3 25 75 100 4 Paper VIII Plant tissue culture 5 3 25 75 100 4 Elective II – Horticulture (Without practical Examination) 5 3 25 75 100 4 Practical - I (Papers I to IV) 5 3 40 60 100 4 Practical - II (Papers V to VIII) 5 3 40 60 100 4 III Paper IX Plant Taxonomy 5 3 25 75 100 4 Paper X Medicinal Botany 5 3 25 75 100 4 Paper XI Plant Physiology 5 3 25 75 100 4 Paper XII Phytochemistry 5 3 25 75 100 4 Elective-III Bioinstrumentation and Biological Techniques (Without practical Examination) 5 3 25 75 100 4 IV Paper XIII Biotechnology and Genetic Engineering 5 3 25 75 100 4 Elective IV-Bioinformatics and Biostatistics (Without practical Examination) 5 3 25 75 100 4 Practical - III (Papers IX & X) 5 3 40 60 100 4 Practical - IV (Papers XI, XII & XIII) 5 3 40 60 100 4 *Project &Viva voce Examination 10 - - - 150 6 Total 2250 90 * Project report – 120 marks; Viva-voce – 30 marks M.Sc Botany-2017-18 onwards – colleges Annexure No:19A Page 2 of 43 Date:03.07.2017 Method of implementation and evaluation of Project Based on the strength, students will be allotted to staff members by lot in the first week after reopening the college. -

Natural Hybridization – Recombination – an Ever-Ongoing Process
THAI FOREST BULL., BOT. 47(1): 19–28. 2019. DOI https://doi.org/10.20531/tfb.2019.47.1.05 Natural hybridization – recombination – an ever-ongoing process NIELS JACOBSEN1,* & MARIAN ØRGAARD1 ABSTRACT Exemplified by studies of the SE Asian genusCryptocoryne (Araceae) we provide evidence that: 1) interspecific hybridization is an ever- ongoing process, and introgression and gene exchange takes place whenever physically possible throughout the region; 2) artificial hybridization experiments confirm that wide crosses are possible in a large number of cases; 3) rivers and streams provide numerous, diverse habitats for Cryptocoryne diaspores to settle in; 4) the changes in habitats caused by recurrent glaciations resulting in numerous splitting and merging of populations facilitates hybridization and segregation of subsequent generations; 5) hybridization is a major driving element in speciation; 6) populations are the units and stepping stones in evolution – not the species. KEYWORDS: Araceae, Chromosome numbers, Cryptocoryne, hybridization, evolution. Accepted for publication: 11 January 2019. Published online: 14 February 2019 INTRODUCTION Chiang Khan, Loei Province, hosts a number of morphologically distinct but genetically closely The completion of Cryptocoryne Fisch. ex related hybrids (Idei et al., 2017). Wydler for the Araceae volume for Flora of Thailand stands as a milestone after several years of research in this biological unique and complex genus (Boyce THE GENUS CRYPTOCORYNE et al., 2012). Numerous cultivation experiments, The genus -

History and Current Status of Systematic Research with Araceae
HISTORY AND CURRENT STATUS OF SYSTEMATIC RESEARCH WITH ARACEAE Thomas B. Croat Missouri Botanical Garden P. O. Box 299 St. Louis, MO 63166 U.S.A. Note: This paper, originally published in Aroideana Vol. 21, pp. 26–145 in 1998, is periodically updated onto the IAS web page with current additions. Any mistakes, proposed changes, or new publications that deal with the systematics of Araceae should be brought to my attention. Mail to me at the address listed above, or e-mail me at [email protected]. Last revised November 2004 INTRODUCTION The history of systematic work with Araceae has been previously covered by Nicolson (1987b), and was the subject of a chapter in the Genera of Araceae by Mayo, Bogner & Boyce (1997) and in Curtis's Botanical Magazine new series (Mayo et al., 1995). In addition to covering many of the principal players in the field of aroid research, Nicolson's paper dealt with the evolution of family concepts and gave a comparison of the then current modern systems of classification. The papers by Mayo, Bogner and Boyce were more comprehensive in scope than that of Nicolson, but still did not cover in great detail many of the participants in Araceae research. In contrast, this paper will cover all systematic and floristic work that deals with Araceae, which is known to me. It will not, in general, deal with agronomic papers on Araceae such as the rich literature on taro and its cultivation, nor will it deal with smaller papers of a technical nature or those dealing with pollination biology. -

Proposed Authorized Plant List by Family
DRAFT Greenhouse Certification Program Authorized Plant List Plants must be propagated from Plants must be seed, tissue culture, Genus contains exclusively or other low risk Not for export to CITES regulated Family Genus Common Names greenhouse-grown plant material Hawaii species Comments Acanthaceae ACANTHUS ZEBRA PLANT, Acanthaceae APHELANDRA 00000 SAFFRON SPIKE Acanthaceae BARLERIA 000000 Acanthaceae CHAMAERANTHEMUM 000000 FIRECRACKER Acanthaceae CROSSANDRA 00000 FLOWER Acanthaceae DICLIPTERA FOLDWING 00000 Acanthaceae FITTONIA MOSAIC PLANT 00000 Acanthaceae GRAPTOPHYLLUM 000000 METAL LEAF, RED Acanthaceae HEMIGRAPHIS IVY, PURPLE 00000 WAFFLE PLANT RIBBON BUSH, Acanthaceae HYPOESTES 00000 POLKA DOT SHRIMP PLANT, BRAZILIAN PLUME, Acanthaceae JUSTICIA 00000 MEXICAN HONEYSUCKLE Acanthaceae ODONTONEMA 000000 Acanthaceae PACHYSTACHYS 000000 Acanthaceae PORPHRYCOMA 000000 Acanthaceae PSEUDERANTHEMUM 000000 Acanthaceae RUELLIA WILD PETUNIA 00000 Acanthaceae SANCHEZIA 000000 Acanthaceae STROBILANTHES 000000 Acanthaceae THUNBERGIA CLOCK VINE 00000 Actinopteridaceae ACTINIOPTERIS 000000 Adiantaceae ADIANTUM MAIDENHAIR FERN 00000 CLOAK FERN, LIP Adiantaceae CHEILANTHES 00000 FERN Adiantaceae HEMIONITIS HEART FERN 00000 DRAFT GCP Authorized Plant List (09/2010) Page 1 of 35 Plants must be propagated from Plants must be seed, tissue culture, Genus contains exclusively or other low risk Not for export to CITES regulated Family Genus Common Names greenhouse-grown plant material Hawaii species Comments CLIFF BRAKE, Adiantaceae PELLAEA 00000 FALCATA