Equus Hydruntinus: Mammalia, Equidae) Revealed by Morphological and Genetical Analyses of Fossils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando
Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando To cite this version: Pablo Librado, Ludovic Orlando. Genomics and the Evolutionary History of Equids. Annual Review of Animal Biosciences, Annual Reviews, 2021, 9 (1), 10.1146/annurev-animal-061220-023118. hal- 03030307 HAL Id: hal-03030307 https://hal.archives-ouvertes.fr/hal-03030307 Submitted on 30 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Anim. Biosci. 2021. 9:X–X https://doi.org/10.1146/annurev-animal-061220-023118 Copyright © 2021 by Annual Reviews. All rights reserved Librado Orlando www.annualreviews.org Equid Genomics and Evolution Genomics and the Evolutionary History of Equids Pablo Librado and Ludovic Orlando Laboratoire d’Anthropobiologie Moléculaire et d’Imagerie de Synthèse, CNRS UMR 5288, Université Paul Sabatier, Toulouse 31000, France; email: [email protected] Keywords equid, horse, evolution, donkey, ancient DNA, population genomics Abstract The equid family contains only one single extant genus, Equus, including seven living species grouped into horses on the one hand and zebras and asses on the other. In contrast, the equine fossil record shows that an extraordinarily richer diversity existed in the past and provides multiple examples of a highly dynamic evolution punctuated by several waves of explosive radiations and extinctions, cross-continental migrations, and local adaptations. -
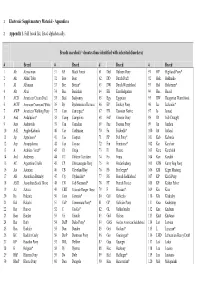
Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

Treatment of Equine Sarcoid with the Mistletoe Extract ISCADOR® P
Pferdeklinik der Vetsuisse-Fakultät der Universität Bern (Leiter: PD. Dr. V. Gerber) Arbeit unter der Leitung von PD Dr. V. Gerber und Prof. Dr. R. Straub Treatment of Equine Sarcoid with the mistletoe extract ISCADOR® P (viscum album austriacus) - a double-blind placebo controlled study Inaugural-Dissertation zur Erlangung der Doktorwürde der Vetsuisse-Fakultät der Universität Bern vorgelegt von Ophélie Clottu von Cornaux und Neuchâtel 2008 1 Von der Vetsuisse-Fakultät der Universität Bern auf Antrag von Prof. Dr. R. Straub als Dissertation genehmigt. Bern, den Der Dekan der Vetsuisse-Fakultät der Universität Bern 2 Treatment of Equine Sarcoid with the mistletoe extract ISCADOR® P (viscum album austriacus) - a double-blind placebo controlled study Summary Background: The Equine Sarcoid (ES) is the most common skin tumour in horses. It is difficult to treat: recurrence rate is high and no single universally effective treatment for ES is known. Viscum album extracts (VAE) are used as adjunct in the treatment of some human cancers without known side effects. Hypothesis: We hypothesized that the long-term therapy with VAE (Iscador® P) is effective in Equine Sarcoids as a single treatment or as an adjunct treatment to selective surgical excision. Material and methods: Of 53 horses with ES, 42 were treated solely with VAE as monotherapy and 11 were treated after selective excision of ES. Horses were randomly assigned to the treatment group (VAE; n=32) or control group (Placebo; n=21). The horses received rising concentrations of Iscador® P extract from 0.1 mg per ml to 20 mg per ml or physiological NaCl solution 3 times a week over 105 days s.c. -

Dietary Adaptability of Late Pleistocene Equus from West Central Mexico Alejandro H. Marín-Leyva , Daniel Demiguel , María
Manuscript Click here to download Manuscript: Manuscript PALAEO8660_Marn Leyva et al _clean version.docClick here to view linked References 1 Dietary adaptability of Late Pleistocene Equus from West Central Mexico 2 3 Alejandro H. Marín-Leyva a,*, Daniel DeMiguel b,*, María Luisa García-Zepeda a, Javier 4 Ponce-Saavedra c, Joaquín Arroyo-Cabrales d, Peter Schaaf e, María Teresa Alberdi f 5 6 a Laboratorio de Paleontología, Facultad de Biología, Universidad Michoacana de San 7 Nicolás de Hidalgo. Edif. R 2°. Piso. Ciudad Universitaria, C.P. 58060, Morelia, 8 Michoacán, Mexico. 9 b Institut Català de Paleontologia Miquel Crusafont (ICP), Edifici Z, Campus de la UAB, C/ 10 de las Columnes s/n, 08193 Cerdanyola del Vallès, Barcelona, Spain 11 c Laboratorio de Entomología “Biol. Sócrates Cisneros Paz”, Facultad de Biología, 12 Universidad Michoacana de San Nicolás de Hidalgo. Edif. B4 2º. Piso. Ciudad 13 Universitaria. C.P. 58060, Morelia, Michoacán, Mexico. 14 d Laboratorio de Arqueozoología „„M. en C. Ticul Álvarez Solórzano‟‟, Subdirección de 15 laboratorio y Apoyo Académico, Instituto Nacional de Antropología e Historia, Moneda 16 #16, Col. Centro, 06060 D.F., Mexico. 17 e Instituto de Geofísica, Universidad Nacional Autónoma de México, Circuito de la 18 Investigación Científica S/N, Ciudad Universitaria, Del. Coyoacán, 04150 México, D.F., 19 México. 20 f Departamento de Paleobiología, Museo Nacional de Ciencias Naturales, CSIC, José 21 Gutiérrez Abascal, 2, 28006 Madrid, Spain. 22 23 *[email protected], [email protected] 1 24 Abstract 25 Most of the Pleistocene species of Equus from Mexico have been considered to be grazers and 26 highly specialized on the basis of their craniodental features, and therefore analogous to their 27 modern relatives from an ecological point of view. -

Sedation and Anesthesia in Military Horses and Mules. Review And
S S E E L L C C Sedation and Anesthesia in Military Horses and Mules. I I T T R R Review and Use in the Swiss Armed Forces. A A By S. MONTAVON∑, C. BAUSSIERE∏, C. SCHMOCKER∏ and M. STUCKI∏. Switzerland Stéphane MONTAVON Colonel Stéphane MONTAVON has been the Chief Veterinary Officer of the Swiss Army since 2003. He obtained his veterinary degree and completed his doctoral thesis at the Vetsuisse veterinary faculty in Bern. He was successively head of clinic at the Federal Army Horse Reserve in Bern, head of clinic at the National Stud in Avenches, resident and clinician at the Veterinary Medical Teaching Hospital at UC Davis (USA). He is a long-time member of the American Association of Equine Practitioners (AAEP). He specializes in equine sports medicine and is an excellent rider and an official veterinarian of the (FEI) Federation Equestre Internationale. RESUME Sédation et anesthésie chez les chevaux et mules militaires. Revue et utilisation dans l'armée suisse. L'armée suisse utilise depuis plus de 100 ans différents types de chevaux pour des missions spéciales. Trois races sont utilisées : Les chevaux de race demi-sang suisse pour l'équitation, les chevaux Franches-Montagnes et les mulets comme « chevaux de bât » pour le transport. Cet article passe en revue les substances, dosages et voies d'administration qui permettent de réaliser des sédations et des anesthésies simples chez le cheval comme sur le mulet, dans le terrain et dans un contexte militaire. Cette contribution est aussi destinée aux autres armées qui utilisent des mulets dans la mesure où la littérature n'est pas très riche dans ce domaine. -

Survey of Risk Factors and Genetic Characterization of Ewe Neck in a World Population of Pura Raza Español Horses
animals Article Survey of Risk Factors and Genetic Characterization of Ewe Neck in a World Population of Pura Raza Español Horses María Ripolles 1, María J. Sánchez-Guerrero 1,2,*, Davinia I. Perdomo-González 1 , Pedro Azor 1 and Mercedes Valera 1 1 Department of Agro-Forestry Sciences, ETSIA, University of Seville, Carretera de Utrera Km 1, 41013 Sevilla, Spain; [email protected] (M.R.); [email protected] (D.I.P.-G.); [email protected] (P.A.); [email protected] (M.V.) 2 Department of Molecular Biology and Biochemistry Engineering, Universidad Pablo de Olavide, Carretera de Utrera Km 1, 41013 Sevilla, Spain * Correspondence: [email protected]; Tel.: +34-9-5448-6461 Received: 31 July 2020; Accepted: 27 September 2020; Published: 1 October 2020 Simple Summary: Ewe Neck is a common morphological defect of the Pura Raza Español (PRE) population, which seriously affects the horse’s development. In this PRE population (35,267 PRE), a total of 9693 animals (27.12% of total) was Ewe Neck-affected. It has been demonstrated that genetic and risk factors (sex, age, geographical area, coat color, and stud size) are involved, being more prevalent in the males, 4–7 years old, chestnut coat, from small studs (less than 5 mares), and raised in North America. The morphological traits height at chest, length of back, head-neck junction, and bottom neck-body junction and the body indices, head index, and thoracic index were those most closely related with the appearance of this morphological defect. The additional genetic base of Ewe Neck in PRE, which presents low-moderate heritability (h2: 0.23–0.34), shows that the prevalence of this defect could be effectively reduced by genetic selection. -

Selected Readings on the History and Use of Old Livestock Breeds
NATIONAL AGRICULTURAL LIBRARY ARCHIVED FILE Archived files are provided for reference purposes only. This file was current when produced, but is no longer maintained and may now be outdated. Content may not appear in full or in its original format. All links external to the document have been deactivated. For additional information, see http://pubs.nal.usda.gov. Selected Readings on the History and Use of Old Livestock Breeds United States Department of Agriculture Selected Readings on the History and Use of Old Livestock Breeds National Agricultural Library September 1991 Animal Welfare Information Center By: Jean Larson Janice Swanson D'Anna Berry Cynthia Smith Animal Welfare Information Center National Agricultural Library U.S. Department of Agriculture And American Minor Breeds Conservancy P.O. Box 477 Pittboro, NC 27312 Acknowledgement: Jennifer Carter for computer and technical support. Published by: U. S. Department of Agriculture National Agricultural Library Animal Welfare Information Center Beltsville, Maryland 20705 Contact us: http://awic.nal.usda.gov/contact-us Web site: www.nal.usda.gov/awic Published in cooperation with the Virginia-Maryland Regional College of Veterinary Medicine Policies and Links Introduction minorbreeds.htm[1/15/2015 2:16:51 PM] Selected Readings on the History and Use of Old Livestock Breeds For centuries animals have worked with and for people. Cattle, goats, sheep, pigs, poultry and other livestock have been an essential part of agriculture and our history as a nation. With the change of agriculture from a way of life to a successful industry, we are losing our agricultural roots. Although we descend from a nation of farmers, few of us can name more than a handful of livestock breeds that are important to our production of food and fiber. -

Comparison of the Interior Characteristics of Slovak Warmblood
Original scientific paper DOI: /10.5513/JCEA01/19.2.2153 Journal of Central European Agriculture, 2018, 19(2), p.295-307 Comparison of the interior characteristics of Slovak warmblood horses and Lipizzan horses Porovnanie interiérových vlastností koní slovenského teplokrvníka a lipicana Michaela HORNÁ*, Marko HALO, Eva MLYNEKOVÁ and Monika IVANČÍKOVÁ Department of Animal Husbandry, Faculty of Agrobiology and Food Resources, Slovak University of Agriculture in Nitra, Nitra, Slovak Republic, *correspondence: [email protected] Abstract One of the important factors that significantly affects the intensity and content of training and determines the performance it the horse interior. Character and temperament traits are described in the breeding goals of each breed of horse. The aim of this study was to analyze the horse interior and comparison the interior characteristics of two studied horse breeds Slovak warmblood horses and Lipizzan horses. Into the analysis were included 65 horses from National Stud Farm Topoľčianky, Slovak warmblood horses (n1 = 33) and Lipizzan horses (n2 = 32).Interior was analyzed through a questionnaire with 13 indicators with 10 point system, where 10 being the highest rating. Slovak warmblood horses become the highest ratings (average 89.1 pt, 74.42%) and Lipizzan horses (86.1 pt, 66.2%), so both breeds of horses can be considered as the breeds with balanced characteristics of temperament and character. But Lipizzan horses are more balanced, because their results had lower variability. There were insignificant differences between the analyzed horses in the studied breeds. Nevertheless, a several analyzes of individual indicators showed that between the Slovak warmblood horses and Lipizzan horses there is a significant difference in a single indicator of interior - 10 - Stress managing. -

Polymorphisms of Coat Color Gene in Jeju Native Horse by Routine Genotyping
저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다. l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer A Thesis For the Degree of Master of Science in Veterinary Medicine Polymorphisms of Coat Color Gene in Jeju Native Horse by Routine Genotyping Department of Veterinary Medicine GRADUATE SCHOOL JEJU NATIONAL UNIVERSITY Yong-Woo Sohn 2010. 12 Polymorphisms of Coat Color Gene in Jeju Native Horse by Routine Genotyping Yong-Woo Sohn (Supervised by Professor Youngmin Yun) A thesis submitted in fulfillment of the requirement for the Degree of Master of Science in Veterinary Medicine 2010. 12. This thesis has been examined and approved by Thesis Director, Kyoungkap Lee, Professor of Veterinary Medicine Gui-Cheol Choi, Adjunct Professor of Veterinary Medicine Chung Buk National University Youngmin Yun, Professor of Veterinary Medicine Department of Veterinary Medicine GRADUATE SCHOOL JEJU NATIONAL UNIVERSITY Polymorphisms of Coat Color Gene in Jeju Native Horse by Routine Genotyping Yong-Woo Sohn (Supervised by Professor Youngmin Yun) Department of Veterinary Medicine, Graduate School, Jeju National University Abstracts Phenotypes of equine coat color depend on the relative amount of eumelanin (black-brown) and phaeomelanin (red-yellow), which are controlled, by the melanocortin-1 receptor (MC1R) and the agouti-signaling peptide (ASIP) genes. -

Humanity from African Naissance to Coming Millennia” Arises out of the World’S First G
copertina2 12-12-2000 12:55 Seite 1 “Humanity from African Naissance to Coming Millennia” arises out of the world’s first J. A. Moggi-Cecchi Doyle G. A. Raath M. Tobias V. P. Dual Congress that was held at Sun City, South Africa, from 28th June to 4th July 1998. “Dual Congress” refers to a conjoint, integrated meeting of two international scientific Humanity associations, the International Association for the Study of Human Palaeontology - IV Congress - and the International Association of Human Biologists. As part of the Dual Congress, 18 Colloquia were arranged, comprising invited speakers on human evolu- from African Naissance tionary aspects and on the living populations. This volume includes 39 refereed papers from these 18 colloquia. The contributions have been classified in eight parts covering to Coming Millennia a wide range of topics, from Human Biology, Human Evolution (Emerging Homo, Evolving Homo, Early Modern Humans), Dating, Taxonomy and Systematics, Diet, Brain Evolution. The book offers the most recent analyses and interpretations in diff rent areas of evolutionary anthropology, and will serve well both students and specia- lists in human evolution and human biology. Editors Humanity from African Humanity Naissance from to Coming Millennia Phillip V. Tobias Phillip V. Tobias is Professor Emeritus at the University of the Witwatersrand, Johannesburg, where he Michael A. Raath obtained his medical doctorate, PhD and DSc and where he served as Chair of the Department of Anatomy for 32 years. He has carried out researches on mammalian chromosomes, human biology of the peoples of Jacopo Moggi-Cecchi Southern Africa, secular trends, somatotypes, hominin evolution, the history of anatomy and anthropology. -

Equus Capensis (Mammalia, Perissodactyla) from Elandsfontein
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE Palaeont. afr., 36, 91-96 (2000) EQUUS CAPENSIS (MAMMALIA, PERISSODACTYLA) FROM ELANDSFONTEIN by Vera Eisenmann Museum National D’ Histoire Naturelle URA 12 and 1415 du CNRS, 8 rue Bujfon, 75005 Paris, France. ABSTRACT The skull and limb bones collected at Elandsfontein, Cape indicate that£. capensis was different from a Grevy's zebra. The body proportions were similar to those of an extant draft horse (E. caballus) and the skull resembled those of true Cape quaggas and a fossil Algerian plains zebra, E. mauritanicus. KEYWORDS: Pleistocene, Elandsfontein, Equus capensis, zebras. INTRODUCTION African Museum Cape Town (E21025). It is very Because Equus capensis is a large equid and because large, but quite unlike a Grevy’s zebra skull. Grevy’s the Grevy's zebra is the largest of extant wild equids, it zebras have very long distances between the posterior has sometimes been considered that they were border of the palate and the posterior border of the conspecific (Churcher & Richardson 1978; Churcher vomer, and their muzzles are narrow (Eisenmann 1980 1986, 1993). This preliminary paper intends to point Plate 1). In the skull of E. capensis mentioned above, out some of the general features of E. capensis which, the muzzle is much wider and the distance between as noted by Broom (1913) “was more powerfully built palate and vomer (vomerine length) is relatively short. but did not stand so high” as “a modem horse 15 hands A scatter diagram of these dimensions in Grevy’s in high”.