Laboratory Activity Histology for Student
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
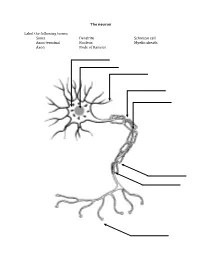
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Cell Biology and Fundamentals in Histology - En-Cours-2018-Liepr1004 Liepr1004 Cell Biology and Fundamentals in 2018 Histology
Université catholique de Louvain - Cell biology and fundamentals in histology - en-cours-2018-liepr1004 liepr1004 Cell biology and fundamentals in 2018 histology 5 credits 45.0 h Q2 Teacher(s) Behets Wydemans Catherine ;Henriet Patrick ; Language : French Place of the course Louvain-la-Neuve Main themes The major themes are : - Characteristics common to all living species - The human cell, its functioning and division - Classical, evolutive and molecular genetics - Cellular bases in sexual reproduction - The differents cell types and their organisation in tissues - The major steps in human embryonic development Aims By the end of the module, students should understand the bases of unicity and diversity in the living world. They will know the structure and functioning of human cell and genome as well as the mechanisms of 1 cell division and embryonic development. Moreover, they will know the structure of the major types of human tissues. - - - - The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”. Content (auteurs - titulaires actuels) : P. Henriet and Ph. van den Bosch de Aguilar 1. UNICITY IN THE LIVING WORLD 2. THE HUMAN CELL 3. DIVERSITY IN THE LIVING WORLD 4. MOLECULAR GENETICS 5. CELL DIVISION 6. GAMETOGENESIS AND FERTILIZATION 7. INTRODUCTION TO HUMAN EMBRYOLOGY Histology 1. EPITHELIAL TISSUE 2. CONNECTIVE TISSUE 3. BLOOD TISSUE 4. MUSCLE TISSUE -
![Matthias Jacob Schleiden (1804–1881) [1]](https://docslib.b-cdn.net/cover/6121/matthias-jacob-schleiden-1804-1881-1-116121.webp)
Matthias Jacob Schleiden (1804–1881) [1]
Published on The Embryo Project Encyclopedia (https://embryo.asu.edu) Matthias Jacob Schleiden (1804–1881) [1] By: Parker, Sara Keywords: cells [2] Matthias Jacob Schleiden helped develop the cell theory in Germany during the nineteenth century. Schleiden studied cells as the common element among all plants and animals. Schleiden contributed to the field of embryology [3] through his introduction of the Zeiss [4] microscope [5] lens and via his work with cells and cell theory as an organizing principle of biology. Schleiden was born in Hamburg, Germany, on 5 April 1804. His father was the municipal physician of Hamburg. Schleiden pursued legal studies at the University of Heidelberg [6] in Heidelberg, Germany, and he graduated in 1827. He established a legal practice in Hamburg, but after a period of emotional depression and an attempted suicide, he changed professions. He studied natural science at the University of Göttingen in Göttingen, Germany, but transferred to the University of Berlin [7] in Berlin, Germany, in 1835 to study plants. Johann Horkel, Schleiden's uncle, encouraged him to study plant embryology [3]. In Berlin, Schleiden worked in the laboratory of zoologistJ ohannes Müller [8], where he met Theodor Schwann. Both Schleiden and Schwann studied cell theory and phytogenesis, the origin and developmental history of plants. They aimed to find a unit of organisms common to the animal and plant kingdoms. They began a collaboration, and later scientists often called Schleiden and Schwann the founders of cell theory. In 1838, Schleiden published "Beiträge zur Phytogenesis" (Contributions to Our Knowledge of Phytogenesis). The article outlined his theories of the roles cells played as plants developed. -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Catholic Christian Christian
Religious Scientists (From the Vatican Observatory Website) https://www.vofoundation.org/faith-and-science/religious-scientists/ Many scientists are religious people—men and women of faith—believers in God. This section features some of the religious scientists who appear in different entries on these Faith and Science pages. Some of these scientists are well-known, others less so. Many are Catholic, many are not. Most are Christian, but some are not. Some of these scientists of faith have lived saintly lives. Many scientists who are faith-full tend to describe science as an effort to understand the works of God and thus to grow closer to God. Quite a few describe their work in science almost as a duty they have to seek to improve the lives of their fellow human beings through greater understanding of the world around them. But the people featured here are featured because they are scientists, not because they are saints (even when they are, in fact, saints). Scientists tend to be creative, independent-minded and confident of their ideas. We also maintain a longer listing of scientists of faith who may or may not be discussed on these Faith and Science pages—click here for that listing. Agnesi, Maria Gaetana (1718-1799) Catholic Christian A child prodigy who obtained education and acclaim for her abilities in math and physics, as well as support from Pope Benedict XIV, Agnesi would write an early calculus textbook. She later abandoned her work in mathematics and physics and chose a life of service to those in need. Click here for Vatican Observatory Faith and Science entries about Maria Gaetana Agnesi. -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Immune Response and Histology of Humoral Rejection in Kidney
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):354–367 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Review Immune response and histology of humoral rejection in kidney transplantation a,∗ a b a Miguel González-Molina , Pedro Ruiz-Esteban , Abelardo Caballero , Dolores Burgos , a c a a Mercedes Cabello , Miriam Leon , Laura Fuentes , Domingo Hernandez a Nephrology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain b Immunology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain c Pathology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain a r t i c l e i n f o a b s t r a c t Article history: The adaptive immune response forms the basis of allograft rejection. Its weapons are direct Received 4 June 2015 cellular cytotoxicity, identified from the beginning of organ transplantation, and/or anti- Accepted 26 March 2016 bodies, limited to hyperacute rejection by preformed antibodies and not as an allogenic Available online 3 June 2016 response. This resulted in allogenic response being thought for decades to have just a cellu- lar origin. But the experimental studies by Gorer demonstrating tissue damage in allografts Keywords: due to antibodies secreted by B lymphocytes activated against polymorphic molecules were Immune response disregarded. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Chapter 8 Nervous System
Chapter 8 Nervous System I. Functions A. Sensory Input – stimuli interpreted as touch, taste, temperature, smell, sound, blood pressure, and body position. B. Integration – CNS processes sensory input and initiates responses categorizing into immediate response, memory, or ignore C. Homeostasis – maintains through sensory input and integration by stimulating or inhibiting other systems D. Mental Activity – consciousness, memory, thinking E. Control of Muscles & Glands – controls skeletal muscle and helps control/regulate smooth muscle, cardiac muscle, and glands II. Divisions of the Nervous system – 2 anatomical/main divisions A. CNS (Central Nervous System) – consists of the brain and spinal cord B. PNS (Peripheral Nervous System) – consists of ganglia and nerves outside the brain and spinal cord – has 2 subdivisions 1. Sensory Division (Afferent) – conducts action potentials from PNS toward the CNS (by way of the sensory neurons) for evaluation 2. Motor Division (Efferent) – conducts action potentials from CNS toward the PNS (by way of the motor neurons) creating a response from an effector organ – has 2 subdivisions a. Somatic Motor System – controls skeletal muscle only b. Autonomic System – controls/effects smooth muscle, cardiac muscle, and glands – 2 branches • Sympathetic – accelerator “fight or flight” • Parasympathetic – brake “resting and digesting” * 4 Types of Effector Organs: skeletal muscle, smooth muscle, cardiac muscle, and glands. III. Cells of the Nervous System A. Neurons – receive stimuli and transmit action potentials -

Satellitosis, a Crosstalk Between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour
cancers Review Satellitosis, a Crosstalk between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour Prospero Civita 1,2,* , Ortenzi Valerio 3 , Antonio Giuseppe Naccarato 3 , Mark Gumbleton 2 and Geoffrey J. Pilkington 1,2,4,* 1 Brain Tumour Research Centre, Institute of Biological and Biomedical Sciences (IBBS), School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth PO1 2DT, UK 2 School of Pharmacy and Pharmaceutical Sciences, College of Biomedical and Life Sciences, Cardiff University, Cardiff CF10 3NB, UK; gumbleton@cardiff.ac.uk 3 Department of Translational Research and New Technologies in Medicine and Surgery, Pisa University Hospital, 56100 Pisa, Italy; [email protected] (O.V.); [email protected] (A.G.N.) 4 Division of Neuroscience, Department of Basic and Clinical Neuroscience, Institute of Psychiatry & Neurology, King’s College London, London SE5 9RX, UK * Correspondence: CivitaP@cardiff.ac.uk (P.C.); geoff[email protected] (G.J.P.) Received: 9 November 2020; Accepted: 4 December 2020; Published: 11 December 2020 Simple Summary: This article reviews the concept of cellular satellitosis as originally described histologically by Santiago Ramón y Cajal in 1899 and Hans Joachim Scherer, more specifically in the context of glioblastoma invasiveness, during the early part of the 20th century. With the advent of new and emerging molecular technologies in the 21st century, the significance of both vascular and neuronal satellitosis by neoplastic cells offers intriguing possibilities into further clarifying the development, pathobiology and therapy of malignant glioma through closer investigation into the nature of these histological hallmarks. -

Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3
New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. In small neurons (e.g., den- tate granule neurons), excitability was highest when the AIS was of intermediate length and located adjacent to the soma. Conversely, neu- rons having larger dendritic trees (e.g., pyramidal neurons) were most excitable when the AIS was longer and/or located away from the soma. For any given somatodendritic morphology, increasing dendritic mem- brane capacitance and/or conductance favored a longer and more distally located AIS. Overall, changes to AIS length, with corresponding changes in total sodium conductance, were far more effective in regulating neuron excitability than were changes in AIS location, while dendritic capacitance had a larger impact on AIS performance than did dendritic conductance. The somatodendritic influence on AIS performance reflects modest soma- to-AIS voltage attenuation combined with neuron size-dependent changes in AIS input resistance, effective membrane time constant, and isolation from somatodendritic capacitance. -

How Does an Axon Grow?
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW How does an axon grow? Jeffrey L. Goldberg1 Department of Neurobiology, Stanford University School of Medicine, Stanford, California 94305, USA How do axons grow during development, and why do cisions to advance, retract, pause, or turn (Fig. 1). All of they fail to regrow when injured? In the complicated these are potential regulatory sites that could control a mesh of our nervous system, the axon is the information neuron’s ability to elongate or regenerate its axon. superhighway, carrying all of the data we use to sense our environment and carry out behaviors. To wire up our Production of the building blocks, nervous system properly, neurons must elongate their both membrane and cytoplasmic axons during development to reach their targets. This is no simple task, however. The complex morphology of Rapid axon growth requires rapid manufacture and sup- axons and dendrites puts neurons among the most intri- ply of cytoplasm and membrane. Where are the lipid and cate and beautiful cells in the body. Knowledge of how protein building blocks made? It was known from classic neurons extend axons and dendrites, elongate at a par- experiments that axons cut off from the cell bodies of ticular rate, and stop growing at the proper time is criti- adult sensory neurons continue to elongate in culture cal to understanding the development of our nervous (Shaw and Bray 1977), but evidence for local production system, yet the regulation of these processes is poorly of membrane and cytoplasmic elements went lacking for understood.