List of Plant Diseases American Samoa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHAPTER 2 REVIEW of the LITERATURE 2.1 Taxa And
CHAPTER 2 REVIEW OF THE LITERATURE 2.1 Taxa and Classification of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.11 Taxa and Classification of Acalypha indica Linn. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Euphorbiales Family : Euphorbiaceae Subfamily : Acalyphoideae Genus : Acalypha Species : Acalypha indica Linn. (Saha and Ahmed, 2011) Plant Synonyms: Acalypha ciliata Wall., A. canescens Wall., A. spicata Forsk. (35) Common names: Brennkraut (German), alcalifa (Brazil) and Ricinela (Spanish) (36). 9 2.12 Taxa and Classification of Bridelia retusa (L.) A. Juss. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Bridelia Species : Bridelia retusa (L.) A. Juss. Plant Synonyms: Bridelia airy-shawii Li. Common names: Ekdania (37,38). 2.13 Taxa and Classification of Cleidion javanicum BL. Kingdom : Plantae Subkingdom : Tracheobionta Superdivision : Spermatophyta Division : Magnoliophyta Class : Magnoliopsida Subclass : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Cleidion Species : Cleidion javanicum BL. Plant Synonyms: Acalypha spiciflora Burm. f. , Lasiostylis salicifolia Presl. Cleidion spiciflorum (Burm.f.) Merr. Common names: Malayalam and Yellari (39). 10 2.2 Review of chemical composition and bioactivities of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.2.1 Review of chemical composition and bioactivities of Acalypha indica Linn. Acalypha indica -

Genome Sequence Analysis of Auricularia Heimuer Combined with Genetic Linkage Map
Journal of Fungi Article Genome Sequence Analysis of Auricularia heimuer Combined with Genetic Linkage Map Ming Fang 1, Xiaoe Wang 2, Ying Chen 2, Peng Wang 2, Lixin Lu 2, Jia Lu 2, Fangjie Yao 1,2,* and Youmin Zhang 1,* 1 Lab of genetic breeding of edible mushromm, Horticultural, College of Horticulture, Jilin Agricultural University, Changchun 130118, China; [email protected] 2 Engineering Research Centre of Chinese Ministry of Education for Edible and Medicinal Fungi, Jilin Agricultural University, Changchun 130118, China; [email protected] (X.W.); [email protected] (Y.C.); [email protected] (P.W.); [email protected] (L.L.); [email protected] (J.L.) * Correspondence: [email protected] (F.Y.); [email protected] (Y.Z.) Received: 3 March 2020; Accepted: 12 March 2020; Published: 16 March 2020 Abstract: Auricularia heimuer is one of the most popular edible fungi in China. In this study, the whole genome of A. heimuer was sequenced on the Illumina HiSeq X system and compared with other mushrooms genomes. As a wood-rotting fungus, a total of 509 carbohydrate-active enzymes (CAZymes) were annotated in order to explore its potential capabilities on wood degradation. The glycoside hydrolases (GH) family genes in the A. heimuer genome were more abundant than the genes in the other 11 mushrooms genomes. The A. heimuer genome contained 102 genes encoding class III, IV, and V ethanol dehydrogenases. Evolutionary analysis based on 562 orthologous single-copy genes from 15 mushrooms showed that Auricularia formed an early independent branch of Agaricomycetes. The mating-type locus of A. heimuer was located on linkage group 8 by genetic linkage analysis. -
![Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]](https://docslib.b-cdn.net/cover/8673/entry-for-acalypha-acrogyna-pax-family-euphorbiaceae-78673.webp)
Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]
Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] http://plants.jstor.org/flora/flota011327 http://www.jstor.org Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the contributing partner regarding any further use of this work. Partner contact information may be obtained at http://plants.jstor.org/page/about/plants/PlantsProject.jsp. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Page 1 of 2 Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] Herbarium Royal Botanic Gardens, Kew (K) Collection Flora of Tropical Africa Resource Type Reference Sources Entry from Flora of Tropical Africa, Vol 6 Part 1, page 441 (1913) Author: (By J. G. Baker, with additions by C. H. Wright.) Names ACALYPHA acrogyna Pax [family EUPHORBIACEAE], in Engl. Jahrb. -

Species Concepts in Cercospora: Spotting the Weeds Among the Roses
available online at www.studiesinmycology.org STUDIES IN MYCOLOGY 75: 115–170. Species concepts in Cercospora: spotting the weeds among the roses J.Z. Groenewald1*, C. Nakashima2, J. Nishikawa3, H.-D. Shin4, J.-H. Park4, A.N. Jama5, M. Groenewald1, U. Braun6, and P.W. Crous1, 7, 8 1CBS-KNAW Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands; 2Graduate School of Bioresources, Mie University, 1577 Kurima-machiya, Tsu, Mie 514–8507, Japan; 3Kakegawa Research Center, Sakata Seed Co., 1743-2 Yoshioka, Kakegawa, Shizuoka 436-0115, Japan; 4Division of Environmental Science and Ecological Engineering, College of Life Sciences and Biotechnology, Korea University, Seoul 136-701, Korea; 5Department of Agriculture, P.O. Box 326, University of Reading, Reading RG6 6AT, UK; 6Martin-Luther-Universität, Institut für Biologie, Bereich Geobotanik und Botanischer Garten, Herbarium, Neuwerk 21, 06099 Halle (Saale), Germany; 7Microbiology, Department of Biology, Utrecht University, Padualaan 8, 3584 CH Utrecht, the Netherlands; 8Wageningen University and Research Centre (WUR), Laboratory of Phytopathology, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands *Correspondence: Johannes Z. Groenewald, [email protected] Abstract: The genus Cercospora contains numerous important plant pathogenic fungi from a diverse range of hosts. Most species of Cercospora are known only from their morphological characters in vivo. Although the genus contains more than 5 000 names, very few cultures and associated DNA sequence data are available. In this study, 360 Cercospora isolates, obtained from 161 host species, 49 host families and 39 countries, were used to compile a molecular phylogeny. Partial sequences were derived from the internal transcribed spacer regions and intervening 5.8S nrRNA, actin, calmodulin, histone H3 and translation elongation factor 1-alpha genes. -
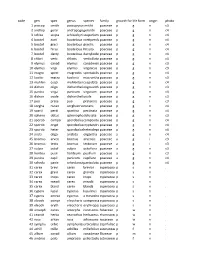
Species List (PDF)
code gen spec genus species family growth formlife form origin photo 1 pascop smith pascopyrumsmithii poaceae p g n c3 2 androp gerar andropogongerardii poaceae p g n c4 3 schiza scopa schizachyriumscoparium poaceae p g n c4 4 boutel curti bouteloua curtipendulapoaceae p g n c4 5 boutel graci bouteloua gracilis poaceae p g n c4 6 boutel hirsu bouteloua hirsuta poaceae p g n c4 7 boutel dacty bouteloua dactyloidespoaceae p g n c4 8 chlori verti chloris verticillata poaceae p g n c4 9 elymus canad elymus canadensispoaceae p g n c3 10 elymus virgi elymus virginicus poaceae p g n c3 11 eragro spect eragrostis spectabilis poaceae p g n c4 12 koeler macra koeleria macrantha poaceae p g n c3 13 muhlen cuspi muhlenbergiacuspidata poaceae p g n c4 14 dichan oligo dichantheliumoligosanthespoaceae p g n c3 15 panicu virga panicum virgatum poaceae p g n c4 16 dichan ovale dichantheliumovale poaceae p g n c3 17 poa prate poa pratensis poaceae p g i c3 18 sorgha nutan sorghastrumnutans poaceae p g n c4 19 sparti pecti spartina pectinata poaceae p g n c4 20 spheno obtus sphenopholisobtusata poaceae p g n c3 21 sporob compo sporoboluscomposituspoaceae p g n c4 22 sporob crypt sporoboluscryptandruspoaceae p g n c4 23 sporob heter sporobolusheterolepispoaceae p g n c4 24 aristi oliga aristida oligantha poaceae a g n c4 25 bromus arven bromus arvensis poaceae a g i c3 26 bromus tecto bromus tectorum poaceae a g i c3 27 vulpia octof vulpia octoflora poaceae a g n c3 28 hordeu pusil hordeum pusillum poaceae a g n c3 29 panicu capil panicum capillare poaceae a g n c4 30 schedo panic schedonnarduspaniculatuspoaceae p g n c4 31 carex brevi carex brevior cyperaceaep s n . -

Alternaria Brassicicola)
Int.J.Curr.Microbiol.App.Sci (2020) 9(8): 2553-2559 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 9 Number 8 (2020) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2020.908.292 In-vivo Management of Alternaria Leaf Spot of Cabbage (Alternaria brassicicola) Dwarkadas T. Bhere*, K. M. Solanke, Amrita Subhadarshini, Shashi Tiwari and Mohan K. Narode Department of Plant Pathology, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, (U. P), India *Corresponding author ABSTRACT K e yw or ds An experiment was conducted for in-vivo management of Alternaria leaf spot of Cabbage. Alternaria leaf spot, The experiment was analyzed by using RBD (randomized block design) with three 2 Cabbage, replications in a plot size 2x2m . Eight treatments were taken i.e. Neem oil, Eucalyptus oil, Eucalyptus oil and Clove oil, Trichoderma viride, Neem oil + Trichoderma viride, Eucalyptus oil + Clove oil, Trichoderma viride, Clove oil + Trichoderma viride along with the control. Observations Trichoderma viride, were recorded at disease intensity 30, 45 and 60 (days after Transplanting), plant growth Neem oil parameters such a yield (q/ha). Experiment revealed that Neem oil significantly reduced the Alternaria leaf spot of Cabbage, where among the use Neem oil seedling treatment @ Article Info 5% increased the yield. The maximum cost benefit ratio was recorded by Neem oil (1:3.26) Thus according to experimental finding and results discussed in the earlier Accepted: chapter, it is concluded that Neem oil reduced the Alternaria leaf spot of Cabbage, where 22 July 2020 among the Neem oil seedling application found maximum yield was significantly superior Available Online: 10 August 2020 as compare to other treatments. -

Infection Cycle of Alternaria Brassicicola on Brassica Oleracea Leaves Under Growth Room Conditions
Plant Pathology (2018) 67, 1088–1096 Doi: 10.1111/ppa.12828 Infection cycle of Alternaria brassicicola on Brassica oleracea leaves under growth room conditions V. K. Macioszeka, C. B. Lawrenceb and A. K. Kononowicza* aDepartment of Genetics, Plant Molecular Biology and Biotechnology, Faculty of Biology and Environmental Protection, University of Lodz, 90-237 Lodz, Poland; and bDepartment of Biological Sciences, Virginia Tech, Blacksburg, VA 24061, USA Development of the necrotrophic fungus Alternaria brassicicola was evaluated during infection of three cabbage vari- eties: Brassica oleracea var. capitata f. alba ‘Stone Head’ (white cabbage), B. oleracea var. capitata f. rubra ‘Langedi- jker Dauer’ (red cabbage) and B. oleracea var. capitata f. sabauda ‘Langedijker Dauerwirsing’ (Savoy cabbage). Following inoculation of cabbage leaves, conidial germination, germ tube growth, and appressorium formation were analysed during the first 24 h of infection. Differences in the dynamics of fungal development on leaves were observed, e.g. approximately 40% of conidia germinated on Savoy cabbage leaves at 4 h post-inoculation (hpi) while only 20% germinated on red and white cabbage leaves. Leaf penetration on the three cabbage varieties mainly occurred through appressoria, rarely through stomata. Formation of infection cushions was found exclusively on red cabbage. Appresso- ria were first observed on red cabbage leaves at 6 hpi, and on white and Savoy cabbage leaves at 8 hpi. Conidiogenesis occurred directly from mature conidia at an early stage of fungal development (10 hpi), but later (48 hpi) it occurred through conidiophores. Disease progress and changes in the morphology of leaf surfaces were also observed. At the final 120 hpi measurement point, necroses on all investigated varieties were approximately the same size. -

Plant-Parasitic Algae (Chlorophyta: Trentepohliales) in American Samoa1
Plant-Parasitic Algae (Chlorophyta: Trentepohliales) in American Samoa1 Fnd E. Erooks 2 Abstract: A survey conducted betweenJune 2000 and May 2002 on the island of Tutuila, American Samoa, recorded filamentous green algae of the order Tren tepohliales (CWorophyta) and their plant hosts. Putative pathogenicity of the parasitic genus Cephaleuros and its lichenized state, Strig;ula, was also inves tigated. Three genera and nine species were identified: Cephaleuros (five spp.), Phycopeltis (two spp.), and Stomatochroon (two spp.). A widely distributed species of Trentepohlia was not classified. These algae occurred on 146 plant species and cultivars in 101 genera and 48 families; 90% of the hosts were dicotyledonous plants. Cephaleuros spp. have aroused worldwide curiosity, confusion, and con cern for over a century. Their hyphaelike filaments, sporangiophores, and as sociated plant damage have led unsuspecting plant pathologists to misidentify them as fungi, and some phycologists question their parasitic ability. Of the five species of Cephaleuros identified, C. virescens was the most prevalent, followed by C. parasiticus. Leaf tissue beneath thalli of Cephaleuros spp. on 124 different hosts was dissected with a scalpel and depth of necrosis evaluated using a four point scale. No injury was observed beneath thalli on 6% of the hosts, but full thickness necrosis occurred on leaves of 43% of hosts. Tissue damage beneath nonlichenized Cephaleuros thalli was equal to or greater than damage beneath lichenized thalli (Strig;ula elegans). In spite of moderate to severe leaf necrosis caused by Cephaleuros spp., damage was usually confined to older leaves near the base of plants. Unhealthy, crowded, poorly maintained plants tended to have the highest percentage of leaf surface area affected by TrentepoWiales. -

A New Species of Bondarzewia from India
Turkish Journal of Botany Turk J Bot (2015) 39: 128-133 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1402-82 A new species of Bondarzewia from India 1, 1 2 Kanad DAS *, Arvind PARIHAR , Manoj Emanuel HEMBROM 1 Botanical Survey of India, Cryptogamic Unit, P. O. B. Garden, Howrah, India 2 Botanical Survey of India, Central National Herbarium, P. O. B. Garden, Howrah, India Received: 25.02.2014 Accepted: 18.07.2014 Published Online: 02.01.2015 Printed: 30.01.2015 Abstract: Bondarzewia zonata, collected from North Sikkim, is proposed here as new to science. It is characterized by basidiomata with strong zonate pilei, thin context turning persistent dark red with guaiacol, comparatively small spores with narrow ornamented ridges, and an absence of cystidioles. A detailed description coupled with macro- and micromorphological illustrations of this species is provided. Its relation to the allied species is discussed and a provisional key to the species of Bondarzewia is given. Key words: Macrofungi, Bondarzewia, Russulales, new species, taxonomy, Sikkim 1. Introduction Picea. After thorough macro- and micromorphological The genusBondarzewia was first described by Singer studies followed by a survey of the literature, it proved to (1940). Presently, it accommodates subtropical (Dai et be new to science. It is proposed as Bondarzewia zonata al., 2010) to temperate and wood-inhabiting parasitic and described here in detail with illustrations. Its relation (causing white rot) poroid macrofungi. Therefore, the with closely related taxa is also discussed. genus Bondarzewia can be characterized as pileate stipitate to substipitate basidiocarps, with a dimitic hyphal system 2. -

Leaf Variegation in Caladium Steudneriifolium (Araceae): a Case of Mimicry?
Evol Ecol (2009) 23:503–512 DOI 10.1007/s10682-008-9248-2 ORIGINAL PAPER Leaf variegation in Caladium steudneriifolium (Araceae): a case of mimicry? Ulf Soltau Æ Stefan Do¨tterl Æ Sigrid Liede-Schumann Received: 27 November 2007 / Accepted: 25 February 2008 / Published online: 6 March 2008 Ó Springer Science+Business Media B.V. 2008 Abstract The leaves of Caladium steudneriifolium (Araceae) of the understorey of a submontane rainforest in the Podocarpus National Park (South East Ecuador, 1,060 m a.s.l.) are plain green or patterned with whitish variegation. Of the 3,413 individual leaves randomly chosen and examined in April 2003, two-thirds were plain green, whereas one third were variegated (i.e., whitish due to absence of chloroplasts). Leaves of both morphs are frequently attacked by mining moth caterpillars. Our BLAST analysis based on Cytochrome-c-Oxidase-subunit-1 sequences suggests that the moth is possibly a member of the Pyraloidea or another microlepidopteran group. It was observed that the variegated leaf zones strongly resemble recent damages caused by mining larvae and therefore may mimic an attack by moth larvae. Infestation was significantly 4–12 times higher for green leaves than for variegated leaves. To test the hypothesis that variegation can be interpreted as mimicry to deter ovipositing moths, we first ruled out the possibility that variegation is a function of canopy density (i.e., that the moths might be attracted or deterred by factors unrelated to the plant). Then plain green leaves were artificially variegated and the number of mining larvae counted after 3 months. -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward. -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D.