Norwegian Journal of Entomology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Green-Tree Retention and Controlled Burning in Restoration and Conservation of Beetle Diversity in Boreal Forests
Dissertationes Forestales 21 Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Esko Hyvärinen Faculty of Forestry University of Joensuu Academic dissertation To be presented, with the permission of the Faculty of Forestry of the University of Joensuu, for public criticism in auditorium C2 of the University of Joensuu, Yliopistonkatu 4, Joensuu, on 9th June 2006, at 12 o’clock noon. 2 Title: Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Author: Esko Hyvärinen Dissertationes Forestales 21 Supervisors: Prof. Jari Kouki, Faculty of Forestry, University of Joensuu, Finland Docent Petri Martikainen, Faculty of Forestry, University of Joensuu, Finland Pre-examiners: Docent Jyrki Muona, Finnish Museum of Natural History, Zoological Museum, University of Helsinki, Helsinki, Finland Docent Tomas Roslin, Department of Biological and Environmental Sciences, Division of Population Biology, University of Helsinki, Helsinki, Finland Opponent: Prof. Bengt Gunnar Jonsson, Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden ISSN 1795-7389 ISBN-13: 978-951-651-130-9 (PDF) ISBN-10: 951-651-130-9 (PDF) Paper copy printed: Joensuun yliopistopaino, 2006 Publishers: The Finnish Society of Forest Science Finnish Forest Research Institute Faculty of Agriculture and Forestry of the University of Helsinki Faculty of Forestry of the University of Joensuu Editorial Office: The Finnish Society of Forest Science Unioninkatu 40A, 00170 Helsinki, Finland http://www.metla.fi/dissertationes 3 Hyvärinen, Esko 2006. Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests. University of Joensuu, Faculty of Forestry. ABSTRACT The main aim of this thesis was to demonstrate the effects of green-tree retention and controlled burning on beetles (Coleoptera) in order to provide information applicable to the restoration and conservation of beetle species diversity in boreal forests. -
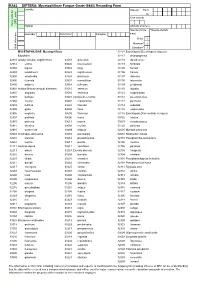
Ra82 Diptera
RA82 DIPTERA: Mycetophilinae Fungus Gnats (6480) Recording Form Locality Date(s) from: to: Vice county GPS users Grey cells for Habitat Altitude (metres) Source (circle *Source details Recorder Determiner Compiler one) Field 1 Museum* 2 Grid reference Literature* 3 MYCETOPHILIDAE: Mycetophilinae 33101 Exechiopsis (Exechiopsis) clypeata Exechiini 33117 dryaspagensis 32801 Allodia (Allodia) anglofennica 33519 griseolum33118 dumitrescae 32912 embla 33526 intermedium33119 fimbriata 32802 lugens 33522 kingi33120 furcata 32803 lundstroemi 33523 nigrofuscum33106 hammi 32804 ornaticollis 33524 proximum33107 indecisa 32806 truncata 33527 rosmellitum33108 intersecta 32805 zaitzevi 33514 ruficorne33109 jenkinsoni 32901 Allodia (Brachycampta) alternans 33515 serenum33110 ligulata 32910 angulata 33516 sericoma33121 magnicauda 32903 barbata 33601 Cordyla brevicornis33112 pseudindecisa 32904 czernyi 33602 crassicornis33113 pulchella 32909 foliifera 33603 fasciata33114 subulata 32905 grata 33604 fissa33115 unguiculata 32906 neglecta 33605 flaviceps33116 Exechiopsis (Xenexechia) crucigera 32907 pistillata 33606 fusca33002 leptura 32915 protenta 33613 insons33003 membranacea 32914 silvatica 33608 murina33122 pollicata 32916 westerholti 33609 nitidula32605 Myrosia maculosa 32602 Allodiopsis domestica 33610 parvipalpis32601 Notolopha cristata 32610 korolevi 33614 pseudomurina32701 Pseudexechia aurivernica 32607 rustica 33611 pusilla32706 monica 32212 Anatella alpina 33612 semiflava32705 parallela 32213 ankeli 33201 Exechia bicincta32703 trisignata 32216 bremia -

Recent Noteworthy Findings of Fungus Gnats from Finland and Northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae)
Biodiversity Data Journal 2: e1068 doi: 10.3897/BDJ.2.e1068 Taxonomic paper Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae) Jevgeni Jakovlev†, Jukka Salmela ‡,§, Alexei Polevoi|, Jouni Penttinen ¶, Noora-Annukka Vartija# † Finnish Environment Insitutute, Helsinki, Finland ‡ Metsähallitus (Natural Heritage Services), Rovaniemi, Finland § Zoological Museum, University of Turku, Turku, Finland | Forest Research Institute KarRC RAS, Petrozavodsk, Russia ¶ Metsähallitus (Natural Heritage Services), Jyväskylä, Finland # Toivakka, Myllyntie, Finland Corresponding author: Jukka Salmela ([email protected]) Academic editor: Vladimir Blagoderov Received: 10 Feb 2014 | Accepted: 01 Apr 2014 | Published: 02 Apr 2014 Citation: Jakovlev J, Salmela J, Polevoi A, Penttinen J, Vartija N (2014) Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae). Biodiversity Data Journal 2: e1068. doi: 10.3897/BDJ.2.e1068 Abstract New faunistic data on fungus gnats (Diptera: Sciaroidea excluding Sciaridae) from Finland and NW Russia (Karelia and Murmansk Region) are presented. A total of 64 and 34 species are reported for the first time form Finland and Russian Karelia, respectively. Nine of the species are also new for the European fauna: Mycomya shewelli Väisänen, 1984,M. thula Väisänen, 1984, Acnemia trifida Zaitzev, 1982, Coelosia gracilis Johannsen, 1912, Orfelia krivosheinae Zaitzev, 1994, Mycetophila biformis Maximova, 2002, M. monstera Maximova, 2002, M. uschaica Subbotina & Maximova, 2011 and Trichonta palustris Maximova, 2002. Keywords Sciaroidea, Fennoscandia, faunistics © Jakovlev J et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Zootaxa, the Fungus Gnats (Diptera: Bolitophilidae
Zootaxa 2318: 450–506 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) The fungus gnats (Diptera: Bolitophilidae, Keroplatidae, Mycetophilidae) of Sardinia, with description of six new species* PETER J. CHANDLER 606B Berryfield Lane, Melksham, Wilts SN12 6EL, United Kingdom. E-mail: [email protected] *In: Cerretti, P., Mason, F., Minelli, A., Nardi, G. & Whitmore, D. (Eds), Research on the Terrestrial Arthropods of Sardinia (Italy). Zootaxa, 2318, 1–602. Table of contents Abstract . .450 Introduction . .451 Study area . .452 Material and methods . .452 Abbreviations . .453 Sampling sites . .454 Faunistic list . .456 Bolitophilidae . .456 Keroplatidae, Keroplatinae, Keroplatini . .456 Orfeliini . .457 Macrocerinae . .462 Mycetophilidae, Gnoristinae . .465 Leiinae . .469 Mycetophilinae, Exechiini . .472 Mycetophilini . .480 Mycomyinae . .492 Sciophilinae . .495 Discussion . .500 Acknowledgements . .501 References . .502 Abstract The fungus gnat fauna of Sardinia is reviewed and data presented for all species recorded. Altogether one species of Bolitophilidae, 16 species of Keroplatidae and 105 species of Mycetophilidae are recognised as occurring in Sardinia. As the bolitophilid and two of the mycetophilid species are represented only by females and are not determined to species level, the total confirmed Sardinian list stands at 119 species. Four species of Keroplatidae and 19 species of Mycetophilidae are new to the total Italian fauna, whereas three species of Keroplatidae and 32 species of Mycetophilidae are newly recorded for the island of Sardinia. Six species are described as new to science: two Keroplatidae (Urytalpa juliae sp. nov., Macrocera nuragica sp. nov.) and four Mycetophilidae (Boletina ichnusa sp. nov., Trichonta sandalyon sp. -

Glimpsing at the Rove Beetle Fauna of Vjosa River, Albania (Coleoptera: Staphylinidae) 307-314 © Zool.-Bot
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Verhandlungen der Zoologisch-Botanischen Gesellschaft in Wien. Frueher: Verh.des Zoologisch-Botanischen Vereins in Wien. seit 2014 "Acta ZooBot Austria" Jahr/Year: 2018 Band/Volume: 155_1 Autor(en)/Author(s): Degasperi Gregor Artikel/Article: Glimpsing at the rove beetle fauna of Vjosa River, Albania (Coleoptera: Staphylinidae) 307-314 © Zool.-Bot. Ges. Österreich, Austria; download unter www.zobodat.at Acta ZooBot Austria 155, 2018, 307–314 Glimpsing at the rove beetle fauna of Vjosa River, Albania (Coleoptera: Staphylinidae) Gregor Degasperi The determination of by-catches from a field trip to Vjosa River in April 2017 revealed 74 different species of staphylinid beetles. 28 species were reported for the first time for Albania, which impressively confirms the poor knowledge of Albania’s rove beetle fauna. Further intensified investigations also considering specific catching methods are recommended to generate a well-founded data set of riverine staphylindae from Vjosa River for detailed evaluation. The importance of staphylindae as indicators in flood- plain habitats is discussed. DEGASPERI G., 2018: Einblicke in die Kurzflügelkäferfauna der Vjosa Auen, Alba- nien (Coleoptera: Staphylinidae). Die Auswertung von Beifängen einer Vjosa Exkursion nach Albanien im April 2017 erbrachten 74 verschiedene Arten. 28 Arten Staphylindae werden zum ersten Mal aus Albanien gemeldet, was den geringen Kenntnisstand der Staphyliniden Fauna Alba- niens demonstriert. Für eine ökologische Auswertung werden zusätzliche und inten- sivere Aufsammlungen unter Anwendung spezifischer Sammelmethoden empfohlen. Die zentrale Bedeutung von Kurzflügelkäfern als Indikatoren in Auen Lebensräumen wird diskutiert. Keywords: Albania, floodplain, Staphylinidae, new records, riparian, river. -

Folia Oecologica
ACTA UNIVERSITATIS PREŠOVIENSIS PRÍRODNÉ VEDY FOLIA OECOLOGICA Ročník 9., číslo 2. Prešov 2017 Časopis je jedným z výsledkov realizácie projektu: „Inovácia vzdelávacieho a výskumného procesu ekológie ako jednej z nosných disciplín vedomostnej spoločnosti“, ITMS: 26110230119, podporeného z operačného programu Vzdelávanie, spolufinancovaného zo zdrojov EÚ. Editor: RNDr. Adriana Eliašová, PhD. Recenzenti: RNDr. Beáta Baranová, PhD. prof. RNDr. Miroslav Barták, CSc. Ing. Lenka Bobuľská, PhD. RNDr. Lukáš Drag, PhD. RNDr. Alena Gajdošová, CSc. doc. Ing. Miroslav Habán, PhD. Dr. Roman J. Hodunko, CSc. doc. RNDr. Ján Kodada, CSc. Mgr. Peter Manko, PhD. Ing. Jozef Oboňa, PhD. Ing. Miroslav Očadlík, PhD. RNDr. Terézia Pošiváková, PhD. RNDr. Radoslav Smoľák, PhD. Ing. Stanislav Torma, PhD. Redakčná rada: Predseda: doc. Mgr. Martin Hromada, PhD. Výkonný redaktor: RNDr. Adriana Eliašová, PhD. Členovia: RNDr. Ema Gojdičová, PhD. Mgr. Tomáš Jászay, PhD. prof. PaedDr. Ján Koščo, PhD. Mgr. Peter Manko, PhD. doc. RNDr. Ivan Šalamon, CSc. doc. RNDr. Marcel Uhrin, PhD. Adresa redakcie: Acta Universitatis Prešoviensis – Folia Oecologica Katedra ekológie FHPV PU Ulica 17. novembra 1, 081 16 Prešov Slovensko Tel: 051 / 75 70 358 e-mail: [email protected] Vydavateľ: Vydavateľstvo Prešovskej univerzity v Prešove Sídlo vydavateľa: Ulica 17. novembra 15, 080 01 Prešov IČO vydavateľa: 17 070 775 Periodicita: 2x ročne Jazyk: slovenský Poradie vydania: 2/2017 Dátum vydania: december 2017 ISSN1338-080X EV 3883/09 OBSAH / CONTENTS Jozef Oboňa – Lenka Demková – Martina Kohútová – Jan Máca – Peter Manko On the occurrence of Drosophila suzukii (Matsumura, 1931) in Slovakia ....................................................................... 5 Libor Dvořák – Jean-Paul Haenni – Jan Máca – Ruslan Mariychuk – Jozef Oboňa Some insects (Dermaptera, Diptera, Mecoptera) from beer traps in Uzhhorod City (Ukraine) ................................................................... -

TORTS Newsletter of the Troop of Reputed Tortricid Systematists ISSN 1945-807X (Print) ISSN 1945-8088 (Online)
Volume 11 14 February 2010 Issue 1 TORTS Newsletter of the Troop of Reputed Tortricid Systematists ISSN 1945-807X (print) ISSN 1945-8088 (online) NEW LEPIDOPTERISTS AT papers to Dr. B.-K. Byun - [email protected]. MAJOR INSTITUTIONS PDFs of 11 papers authored or co-authored by Jozef Razowski (2000-2009) can be found at WORLDWIDE http://species.wikimedia.org/wiki/Tortricidae. And as mentioned in a previous issue of the It’s been a remarkable year for those newsletter, issues of Polskie Pismo young scienstists in the job market seeking a Entomologiczne 2006-2009 also are available career postion in Lepidoptera systematics, on-line at http://pte.au.poznan.pl/ppe/ppe.htm. with positions becoming available at The ______________________________________ Natural History Museum, London, U.K., the Australian National Insect Collection TAXONOMIC ADDITIONS AND (ANIC), Canberra, Australia, and The McGuire Center for Lepidoptera and CHANGES PROPOSED IN 2008 Biodiversity, University of Florida, Gainesville. While a new lepidopterist has Below is a list of the new tortricid taxa been hired at The Natural History Museum, proposed in 2008 (with a few overlooked from potential candidates are still being evaluated previous years), followed by a list of new at ANIC and the McGuire Center. synonyms, new combinations, and mis- Thomas Simonsen, most recently from spellings, followed by the literature that the lab of Felix Sperling at the University of supports the proposed additions and changes. Alberta, Edmonton, Canada, accepted the position in London in late 2009. Stay tuned Acleris for news on the positions in Canberra and Gainesville. nishidai Brown, in Brown & Nishida, 2008 ____________________________________ (Acleris), SHILAP Revista de Lepidoptero- logia 36: 342. -

Smithsonian Miscellaneous Collections Volume 117, Number 3
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 117, NUMBER 3 RELATIONSHIPS OF CERTAIN GENERA OF FUNGUS GNATS OF THE FAMILY MYCETOPHILIDAE BY F. R. SHAW AND M. M. SHAW Amherst, Mass. (Publication 4053) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 27, 1951 Z^t £or5 (g»af(tmore (pv&ee BALTIMOEB, MD., 0. B. A. RELATIONSHIPS OF CERTAIN GENERA OF FUNGUS GNATS OF THE FAMILY MYCETOPHILIDAE By F. R. SHAW and M. M. SHAW i Amherst, Mass. The present study represents a continuation of a preliminary in- vestigation of the possible value of thoracic sclerites in determining the relationships of certain insects. Dr. G. C. Crampton was the first to demonstrate the use of these sclerites as a means of determin- ing the systematic position of insects. In 1925, he published a clas- sical study of the comparative morphology of the thorax of nontipu- loid Nematocera. In 1948 Shaw presented a paper in which he indi- cated the value of thoracic sclerites as an aid in determining the phy- logeny of the Mycetophilidae. Although the number of genera he studied was admittedly small, principles were developed that have been of value in distinguishing the phylogenetic relationships of cer- tain genera. Edwards (1925) was the first to indicate that the structure of thoracic sclerites might be of value in determining generic characters in this group. In his monograph of the British fungus gnats he noted that in certain genera the sclerites differed in form and that such dif- ferences might be of value in separating groups of these insects. 1 We wish to express our thanks to the Society of Sigma Xi for a grant-in- aid that made possible the preparation of the illustrations for this paper. -

Environment for Development: an Ecosystems Assessment of Lake Victoria Basin Environmental and Socio-Economic Status, Trends and Human Vulnerabilities
Environment for Development: An Ecosystems Assessment of Lake Victoria Basin Environmental and Socio-Economic Status, Trends and Human Vulnerabilities Editors: Eric O. Odada Daniel O. Olago Washington O. Ochola PAN-AFRICAN SECRETARIAT Environment for Development: An Ecosystems Assessment of Lake Victoria Basin Environmental and Socio-economic Status, Trends and Human Vulnerabilities Editors Eric O. Odada Daniel O. Olago Washington O. Ochola Copyright 2006 UNEP/PASS ISBN ######### Job No: This publication may be produced in whole or part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. UNEP and authors would appreciate receiving a copy of any publication that uses this report as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing of the United Nations Environmental Programme. Citation: Odada, E.O., Olago, D.O. and Ochola, W., Eds., 2006. Environment for Development: An Ecosystems Assessment of Lake Victoria Basin, UNEP/PASS Pan African START Secretariat (PASS), Department of Geology, University of Nairobi, P.O. Box 30197, Nairobi, Kenya Tel/Fax: +254 20 44477 40 E-mail: [email protected] http://pass.uonbi.ac.ke United Nations Environment Programme (UNEP). P.O. Box 50552, Nairobi 00100, Kenya Tel: +254 2 623785 Fax: + 254 2 624309 Published by UNEP and PASS Cover photograph © S.O. Wandiga Designed by: Development and Communication Support Printed by: Development and Communication Support Disclaimers The contents of this volume do not necessarily reflect the views or policies of UNEP and PASS or contributory organizations. -

Genitalia Van Lepidoptera Prepareren En Afbeelden
Entomobrochure 1 (tweede editie – volledig herwerkt) Genitalia van Lepidoptera prepareren en afbeelden Willy De Prins 2007 Vlaamse Vereniging voor Entomologie – Antwerpen Inhoud 1. Inleiding..................................................................................................................................3 2. Benodigdheden .....................................................................................................................4 2.1. Prepareermateriaal ........................................................................................................4 2.2. Chemicaliën ...................................................................................................................6 3. Prepareermethode.................................................................................................................8 3.1. Verwijderen van het achterlijf.........................................................................................8 3.2. Maceratie .......................................................................................................................8 3.3. Uitprepareren .................................................................................................................9 3.4. Ontwateren ....................................................................................................................9 3.5. Insluiten..........................................................................................................................9 3.6. Alternatieve prepareermethoden -

(Diptera, Nematocera, Mycetophilidae). - Im Druck
© Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Sntomojauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 5, Heft 15 ISSN 0250-4413 Linz, 15-Juli 1984 Mycetophiliden aus Lunz, Niederösterreich (Diptera, Nematocera, Mycetophilidae) Norbert Caspers Abstract As part of a limnological and entomological study using the 'greenhouse method1 (ILLIES 1971) 134 species of fungus gnats (Diptera,Nematocera,Mycetophilidae) were collected near of Lunz (Austria). The list of species is presented below. Additional notes are given on capture dates, on taxonomy and on geographical distribution pat- terns of all remarkable species. Two species in the list are new to science: Phthinia plassmanni sp. n., Exechia repandoides sp.n. Zusammenfassung Als Teil einer limnologisch-entomologischen Studie wurden mit Hilfe der Gewächshaus -Methode (ILLIES 1971) 134 Arten von Pilzmücken {Diptera, Nematocera,Mycetophi- lidae) in der Nähe von Lunz (Österreich) gesammelt. Eine Liste der Arten mit zusätzlichen Bemerkungen über Fang- daten, Taxonomie und Verbreitungsmuster aller bemer- 173 © Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at kenswerten Arten wird vorgestellt. Zwei Arten der Liste sind neu für die Wissenschaft: Phthinia plassmanni sp.n., Exechia repandoides sp.n. Die aquatische Entomofauna des Lunzer Seengebietes ist aufgrund der Ergebnisse jahrzehntelanger, intensiver Sammlungs- und Forschungstätigkeit von Mitarbeitern der "Biologischen Station Lunz" die bestbekannte des gesam- ten alpinen Bereichs. Zu diesem hohen Kenntnisstand -
A Contribution Towards Checklist of Fungus Gnats (Diptera, Diadocidiidae, Ditomyiidae, Bolitophilidae, Keroplatidae, Mycetophilidae) in Georgia, Transcaucasia
ZooKeys 1026: 69–142 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1026.63749 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A contribution towards checklist of fungus gnats (Diptera, Diadocidiidae, Ditomyiidae, Bolitophilidae, Keroplatidae, Mycetophilidae) in Georgia, Transcaucasia Olavi Kurina1 1 Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, Kreutzwaldi st 5 D, 51006 Tartu, Estonia Corresponding author: Olavi Kurina ([email protected]) Academic editor: V. Blagoderov | Received 29 January 2021 | Accepted 8 March 2021 | Published 26 March 2021 http://zoobank.org/05EFF10E-6214-4368-BE47-1AA57A2C38D7 Citation: Kurina O (2021) A contribution towards checklist of fungus gnats (Diptera, Diadocidiidae, Ditomyiidae, Bolitophilidae, Keroplatidae, Mycetophilidae) in Georgia, Transcaucasia. ZooKeys 1026: 69–142. https://doi. org/10.3897/zookeys.1026.63749 Abstract The fungus gnats of Georgia are studied based on 2682 specimens collected from 57 localities during 2011–2019. Altogether, 245 species are recorded including four species of Bolitophilidae, three species of Diadocidiidae, two species of Ditomyiidae, 34 species of Keroplatidae and 202 species of Mycet- ophilidae. 230 and 188 species are recorded from Georgia and the whole of Transcaucasia for the first time, respectively. Three new species –Sciophila georgei sp. nov., Leia katae sp. nov. and Anatella metae sp. nov. – are described including detailed illustrations of the male terminalia. Photographs are provided for an additional 38 species to highlight a variability of their general facies. Combined with earlier pub- lished data, the number of fungus gnat species in Georgia is set at 246. The estimated diversity of fungus gnats in Georgia is calculated using non-parametric methods and discussed with respect to other Western Palaearctic regions.