A Wavefront Division Polarimeter for the Measurements of Solute Concentrations in Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 4 Reflected Light Optics
l CHAPTER 4 REFLECTED LIGHT OPTICS 4.1 INTRODUCTION Light is a form ofelectromagnetic radiation. which may be emitted by matter that is in a suitably "energized" (excited) state (e.g.,the tungsten filament ofa microscope lamp emits light when "energized" by the passage of an electric current). One ofthe interesting consequences ofthe developments in physics in the early part of the twentieth century was the realization that light and other forms of electromagnetic radiation can be described both as waves and as a stream ofparticles (photons). These are not conflicting theories but rather complementary ways of describing light; in different circumstances. either one may be the more appropriate. For most aspects ofmicroscope optics. the "classical" approach ofdescribing light as waves is more applicable. However, particularly (as outlined in Chapter 5) when the relationship between the reflecting process and the structure and composition ofa solid is considered. it is useful to regard light as photons. The electromagnetic radiation detected by the human eye is actually only a very small part of the complete electromagnetic spectrum, which can be re garded as a continuum from the very low energies and long wavelengths characteristic ofradio waves to the very high energies (and shortwavelengths) of gamma rays and cosmic rays. As shown in Figure 4.1, the more familiar regions ofthe infrared, visible light, ultraviolet. and X-rays fall between these extremes of energy and wavelength. Points in the electromagnetic spectrum can be specified using a variety ofenergy or wavelength units. The most com mon energy unit employed by physicists is the electron volt' (eV). -

Optical Properties of Tio2 Based Multilayer Thin Films: Application to Optical Filters
Int. J. Thin Fil. Sci. Tec. 4, No. 1, 17-21 (2015) 17 International Journal of Thin Films Science and Technology http://dx.doi.org/10.12785/ijtfst/040104 Optical Properties of TiO2 Based Multilayer Thin Films: Application to Optical Filters. M. Kitui1, M. M Mwamburi2, F. Gaitho1, C. M. Maghanga3,*. 1Department of Physics, Masinde Muliro University of Science and Technology, P.O. Box 190, 50100, Kakamega, Kenya. 2Department of Physics, University of Eldoret, P.O. Box 1125 Eldoret, Kenya. 3Department of computer and Mathematics, Kabarak University, P.O. Private Bag Kabarak, Kenya. Received: 6 Jul. 2014, Revised: 13 Oct. 2014, Accepted: 19 Oct. 2014. Published online: 1 Jan. 2015. Abstract: Optical filters have received much attention currently due to the increasing demand in various applications. Spectral filters block specific wavelengths or ranges of wavelengths and transmit the rest of the spectrum. This paper reports on the simulated TiO2 – SiO2 optical filters. The design utilizes a high refractive index TiO2 thin films which were fabricated using spray Pyrolysis technique and low refractive index SiO2 obtained theoretically. The refractive index and extinction coefficient of the fabricated TiO2 thin films were extracted by simulation based on the best fit. This data was then used to design a five alternating layer stack which resulted into band pass with notch filters. The number of band passes and notches increase with the increase of individual layer thickness in the stack. Keywords: Multilayer, Optical filter, TiO2 and SiO2, modeling. 1. Introduction successive boundaries of different layers of the stack. The interface formed between the alternating layers has a great influence on the performance of the multilayer devices [4]. -

Chirality and Enantiomers
16.11.2010 Bioanalytics Part 5 12.11.2010 Chirality and Enantiomers • Definition: Optical activity • Properties of enantiomers • Methods: – Polarimetry – Circular dichroism • Nomenclature • Separation of enantiomers • Determination of enantiomeric excess (ee) Determination of absolute configuration • Enantiomeric ratio (E‐value) 1 16.11.2010 Definitions Enantiomers: the two mirror images of a molecule Chirality: non‐superimposable mirror‐images •Depends on the symmetry of a molecule •Point‐symmetry: asymmetric C, Si, S, P‐atoms •Helical structures (protein α‐helix) Quarz crystals snail‐shell amino acids Properties of Enantiomers – Chemical identical – Identical UV, IR, NMR‐Spectra – Differences: • Absorption and refraction of circular polarized light is different – Polarimetry, CD‐spectroscopy • Interaction with other chiral molecules/surfaces is different – Separation of enantiomers on chiral columns (GC, HPLC) 2 16.11.2010 Chiral compounds show optical activity A polarimeter is a device which measures the angle of rotation by passing polarized light through an „optical active“ (chiral) substance. Interaction of light and matter If light enters matter, its intensity (amplitude), polarization, velocity, wavelength, etc. may alter. The two basic phenomena of the interaction of light and matter are absorption (or extinction) and a decrease in velocity. 3 16.11.2010 Interaction of light and matter Absorption means that the intensity (amplitude) of light decreases in matter because matter absorbs a part of the light. (Intensity is the square of amplitude.) Interaction of light and matter The decrease in velocity (i.e. the slowdown) of light in matter is caused by the fact that all materials (even materials that do not absorb light at all) have a refraction index, which means that the velocity of light is smaller in them than in vacuum. -

Polarimetric Detection of Enantioselective Adsorption by Chiral Au Nanoparticles – Effects of Temperature, Wavelength and Size
Nanomaterials and Nanotechnology ARTICLE Polarimetric Detection of Enantioselective Adsorption by Chiral Au Nanoparticles – Effects of Temperature, Wavelength and Size Invited Article Nisha Shukla2, Nathaniel Ondeck1, Nathan Khosla1, Steven Klara1, Alexander Petti1 and Andrew Gellman1* 1 Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, PA, USA 2 Institute of Complex Engineered Systems, Carnegie Mellon University, Pittsburgh, PA, USA * Corresponding author(s) E-mail: [email protected] Received 01 November 2014; Accepted 07 January 2015 DOI: 10.5772/60109 © 2015 The Author(s). Licensee InTech. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract 1. Introduction R- and S-propylene oxide (PO) have been shown to interact The importance of chirality arises from the need of the enantiospecifically with the chiral surfaces of Au nanopar‐ pharmaceutical industry, and other producers of bioactive ticles (NPs) modified with D- or L-cysteine (cys). This compounds, for chemical processes that are enantioselec‐ enantiospecific interaction has been detected using optical tive and thereby produce enantiomerically pure chiral polarimetry measurements made on solutions of the D- or products. [1-4] This is one of the most challenging forms of L-cys modified Au (cys/Au) NPs during addition of chemical synthesis and is critical to the performance of racemic PO. The selective adsorption of one enantiomer of bioactive chiral products. Ingestion of the two enantiomers the PO onto the cys/Au NP surfaces results in a net rotation of a chiral compound by a living organism can result in of light during addition of the racemic PO to the solution. -

Optical Properties of Materials JJL Morton
Electrical and optical properties of materials JJL Morton Electrical and optical properties of materials John JL Morton Part 5: Optical properties of materials 5.1 Reflection and transmission of light 5.1.1 Normal to the interface E z=0 z E E H A H C B H Material 1 Material 2 Figure 5.1: Reflection and transmission normal to the interface We shall now examine the properties of reflected and transmitted elec- tromagnetic waves. Let's begin by considering the case of reflection and transmission where the incident wave is normal to the interface, as shown in Figure 5.1. We have been using E = E0 exp[i(kz − !t)] to describe waves, where the speed of the wave (a function of the material) is !=k. In order to express the wave in terms which are explicitly a function of the material in which it is propagating, we can write the wavenumber k as: ! !n k = = = nk0 (5.1) c c0 where n is the refractive index of the material and k0 is the wavenumber of the wave, were it to be travelling in free space. We will therefore write a wave as the following (with z replaced with whatever direction the wave is travelling in): Ex = E0 exp [i (nk0z − !t)] (5.2) In this problem there are three waves we must consider: the incident wave A, the reflected wave B and the transmitted wave C. We'll write down the equations for each of these waves, using the impedance Z = Ex=Hy, and noting the flip in the direction of H upon reflection (see Figure 5.1), and the different refractive indices and impedances for the different materials. -

Investigation of Dichroism by Spectrophotometric Methods
Application Note Glass, Ceramics and Optics Investigation of Dichroism by Spectrophotometric Methods Authors Introduction N.S. Kozlova, E.V. Zabelina, Pleochroism (from ancient greek πλέον «more» + χρόμα «color») is an optical I.S. Didenko, A.P. Kozlova, phenomenon when a transparent crystal will have different colors if it is viewed from Zh.A. Goreeva, T different angles (1). Sometimes the color change is limited to shade changes such NUST “MISiS”, Russia as from pale pink to dark pink (2). Crystals are divided into optically isotropic (cubic crystal system), optically anisotropic uniaxial (hexagonal, trigonal, tetragonal crystal systems) and optically anisotropic biaxial (orthorhombic, monoclinic, triclinic crystal systems). The greatest change is limited to three colors. It may be observed in biaxial crystals and is called trichroic. A two color change may be observed in uniaxial crystals and called dichroic. Pleochroic is often the term used to cover both (2). Pleochroism is caused by optical anisotropy of the crystals Dichroism can be observed in non-polarized light but in (1-3). The absorption of light in the optically anisotropic polarized light it may be more pronounced if the plane of crystals depends on the frequency of the light wave and its polarization of incident light matches plane of polarization of polarization (direction of the electric vector in it) (3, 4). light that propagates in the crystal—ordinary or extraordinary Generally, any ray of light in the optical anisotropic crystal is wave. divided into two rays with perpendicular polarizations and The difference in absorbance of ray lights may be minor, but different velocities (v1, v2) which are inversely proportional to it may be significant and should be considered both when the refractive indices (n1, n2) (4). -
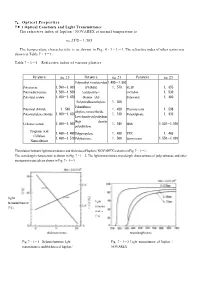
7.1 Optical Constants and Light Transmittance
7 . Optical Properties 7・ 1 Optical Constants and Light Transmittance The refractive index of Iupilon / NOVAREX at normal temperature is nD 25℃ = 1,585 The temperature characteristic is as shown in Fig. 4・1・1‐1. The refractive index of other resins was shown in Table 7・1‐1. Table 7・1‐1 Refractive index of various plastics Polymers nD 25 Polymers nD 25 Polymers nD 25 Polymethyl・methacrylate1.490‐1.500 Polystyrene 1.590‐1.600 (PMMA) 1.570 PETP 1.655 Polymethylstyrene 1.560‐1.580 Acrylonitrile・ 66 Nylon 1.530 Polyvinyl acetate 1.450‐1.470 Styrene(AS Polyacetal 1.480 Polytetrafluoroethylene 1.350 Polutrifluoro Polyvinyl chloride 1.540 1.430 Phenoxy resin 1.598 ethylene monochloride Polyvinylidene chloride 1.600‐1.630 1.510 Polysulphone 1.633 Low density polyethylene High density Cellulose acetate 1.490‐1.500 1.540 SBR 1.520‐1.550 polyethylene Propionic acid 1.460‐1.490 Polypropylene 1.490 TPX 1.465 Cellulose 1.460‐1.510 Polybutyrene 1.500 Epoxy resin 1.550‐1.610 Nitrocellulose The relation between light transmittance and thickness of Iupilon / NOVAREX is shown in Fig. 7・1‐1. The wavelength characteristic is shown in Fig. 7・1‐2. The light transmittance wavelength characteristics of polycarbonate and other transparent materials are shown in Fig. 7・1‐3. light transmittance light (%) transmi- ttance (%) thickness (mm) wavelength (nm) Fig. 7・1‐1 Relation between light Fig. 7・1‐2 Light transmittance of Iupilon / transmittancee and thickness of Iupilon / NOVAREX NOVAREX glass 5.72mm injection molding PMMA 3.43mm compression molding light 3.25mm transmittance injection molding (%) PC 3.43mm wavelength (nm) Fig. -

Polarimeter Buyer's Guide
Polarimeter Buyer’s Guide Understanding Polarimeters Selecting the Right Polarimeter Illumination Method 3 Polarimeters use different lamp sources for transmitting Measurement Range and Accuracy Polarimeters are precise optical instruments that measure purity polarized light. Some models employ halogen lamps which 1 Purchasing a new polarimeter is a major decision. Choose the and concentration through the optical activity exhibited in have a short life span, generate heat, and require servicing model that offers the best measurement range and accuracy inorganic and organic compounds. Compounds are considered while others have LED illumination. LED illumination does not to meet present and future application needs. Be aware to be optically active if linearly polarized light is rotated when require maintenance or generate heat and is environmentally that many polarimeters offer a very limited measurement passing through them. This optical rotation in general means friendly and energy efficient. All Reichert polarimeters feature range that may limit readings. Also, most importantly, there that the polarization of the direction of light is rotated at LED illumination. a specific angle proportionate to the concentration of the are many polarimeters on the market that have a non-linear accuracy specification. In other words, within the limited optically active substance being tested. Maintenance measurement range of these instruments, the accuracy Optical rotation is measured in degrees of angle. At point 4 Operating costs are important to consider when evaluating specification can degrade to different levels across that range. zero, a polarizer and analyzer are set in an angle of 90 degrees instruments. Many companies offer expensive service plans. toward each other, which means that no light reaches the Reichert builds robust instruments that provide reliable detector (0% transmission). -

Radiometry of Light Emitting Diodes Table of Contents
TECHNICAL GUIDE THE RADIOMETRY OF LIGHT EMITTING DIODES TABLE OF CONTENTS 1.0 Introduction . .1 2.0 What is an LED? . .1 2.1 Device Physics and Package Design . .1 2.2 Electrical Properties . .3 2.2.1 Operation at Constant Current . .3 2.2.2 Modulated or Multiplexed Operation . .3 2.2.3 Single-Shot Operation . .3 3.0 Optical Characteristics of LEDs . .3 3.1 Spectral Properties of Light Emitting Diodes . .3 3.2 Comparison of Photometers and Spectroradiometers . .5 3.3 Color and Dominant Wavelength . .6 3.4 Influence of Temperature on Radiation . .6 4.0 Radiometric and Photopic Measurements . .7 4.1 Luminous and Radiant Intensity . .7 4.2 CIE 127 . .9 4.3 Spatial Distribution Characteristics . .10 4.4 Luminous Flux and Radiant Flux . .11 5.0 Terminology . .12 5.1 Radiometric Quantities . .12 5.2 Photometric Quantities . .12 6.0 References . .13 1.0 INTRODUCTION Almost everyone is familiar with light-emitting diodes (LEDs) from their use as indicator lights and numeric displays on consumer electronic devices. The low output and lack of color options of LEDs limited the technology to these uses for some time. New LED materials and improved production processes have produced bright LEDs in colors throughout the visible spectrum, including white light. With efficacies greater than incandescent (and approaching that of fluorescent lamps) along with their durability, small size, and light weight, LEDs are finding their way into many new applications within the lighting community. These new applications have placed increasingly stringent demands on the optical characterization of LEDs, which serves as the fundamental baseline for product quality and product design. -

Chapter 1: Optical Activity and Polarimetry
Chapter 1: Optical Activity and Polarimetry Chiral, non-racemic compounds have the ability to rotate polarized light. This phenomenon is known as circular birefringence, and is where the phrase “optically active” comes from. The specific rotation of a material is an intrinsic, constant value at a given temperature and wavelength of light; specific rotation can be found by using polarimetry. 1.1 Optical Activity Normal light contains a mixture of all vibrational directions that are perpendicular to the direction of travel. We can use polarized filters to block most of the components of normal light and only allow through one direction of polarization (Figure 1-1). Figure 1-1: Polarized light vibrates only in one plane, while normal light vibrates in all planes. When we use two filters in series, the first one is called the polarizer (because it converts the light to polarized light) and the second is called the analyzer (because it allows you to analyze the light you just polarized). If the polarizer and analyzer are parallel to each other, the polarized light gets through fine. But if they’re at right angles, no light can pass through the analyzer, as shown in Figure 1-2. Figure 1-2: Whether polarized light can pass through the analyzer depends on its orientation to the polarizer. Since optical activity gives a compound the ability to rotate plane-polarized light, we can put a sample of the compound in between the polarizer and analyzer. This will rotate the light, allowing some of it to get through the analyzer even when the filters are at right angles (Figure 1-3). -

Schott Filter Catalog
Definitions Glass Filters 2009 OPTICAL FILTERS TABLE OF CONTENTS Table of Contents Foreword ....................................................................................... 4 1. General information on the catalog data ..................................... 5 2. SCHOTT glass filter catalog: product line ..................................... 6 3. Applications for SCHOTT glass filters ............................................ 7 4. Material: filter glass ..................................................................... 10 4.1 Group names ................................................................................ 10 4.2 Classification ................................................................................. 10 4.2.1 Base glasses .................................................................................. 10 4.2.2 Ionically colored glasses ................................................................. 10 4.2.3 Colloidally colored glasses ............................................................. 11 4.3 Reproducibility of transmission ...................................................... 11 4.4 Thermal toughening ..................................................................... 11 5. Filter glass properties .................................................................. 13 5.1 Mechanical density ........................................................................ 13 5.2 Transformation temperature .......................................................... 13 5.3 Thermal expansion ....................................................................... -
Method of Determining the Optical Properties of Ceramics and Ceramic Pigments: Measurement of the Refractive Index
CASTELLÓN (SPAIN) METHOD OF DETERMINING THE OPTICAL PROPERTIES OF CERAMICS AND CERAMIC PIGMENTS: MEASUREMENT OF THE REFRACTIVE INDEX A. Tolosa(1), N. Alcón(1), F. Sanmiguel(2), O. Ruiz(2). (1) AIDO, Instituto tecnológico de Óptica, Color e Imagen, Spain. (2) Torrecid, S.A., Spain. ABSTRACT The knowledge of the optical properties of materials, such as pigments or the ceramics that contain these, is a key feature in modelling the behaviour of light when it impinges upon them. This paper presents a method of obtaining the refractive index of ceramics from diffuse reflectance measurements made with a spectrophotometer equipped with an integrating sphere. The method has been demonstrated to be applicable to opaque ceramics, independently of whether the surface is polished or not, thus avoiding the need to use different methods to measure the refractive index accor- ding to the surface characteristics of the material. This method allows the Fresnel equations to be directly used to calculate the refractive index with the measured diffuse reflectance, without needing to measure just the specular reflectance. Al- ternatively, the method could also be used to measure the refractive index in transparent and translucent samples. The technique can be adapted to determine the complex refractive index of pigments. The reflectance measurement would be based on the method proposed in this paper, but for the calculation of the real part of the refractive index, related to the change in velocity that light undergoes when it reaches the material, and the imaginary part, related to light absorption by the material, the Kramers-Kronig relations would be used.