Radiological and Chemical Properties of Uranium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
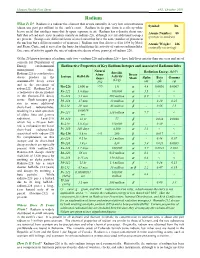
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Chapter 1.2: Transformation Kinetics
Chapter 1: Radioactivity Chapter 1.2: Transformation Kinetics 200 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity http://www.world‐nuclear.org/info/inf30.html 201 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity Serial Transformation In many situations, the parent nuclides produce one or more radioactive offsprings in a chain. In such cases, it is important to consider the radioactivity from both the parent and the daughter nuclides as a function of time. • Due to their short half lives, 90Kr and 90Rb will be completely transformed, results in a rapid building up of 90Sr. • 90Y has a much shorter half‐life compared to 90Sr. After a certain period of time, the instantaneous amount of 90Sr transformed per unit time will be equal to that of 90Y. •Inthiscase,90Y is said to be in a secular equilibrium. 202 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity Exponential DecayTransformation Kinetics • Different isotopes are characterized by their different rate of transformation (decay). • The activity of a pure radionuclide decreases exponentially with time. For a given sample, the number of decays within a unit time window around a given time t is a Poisson random variable, whose expectation is given by t Q Q0e •Thedecayconstant is the probability of a nucleus of the isotope undergoing a decay within a unit period of time. 203 NPRE 441, Principles of Radiation Protection Chapter 1: Radioactivity Why Exponential Decay? The decay rate, A, is given by Separate variables in above equation, we have The decay constant is the probability of a nucleus of the isotope undergoing a decay within a unit period of time. -

FACT SHEET Office of Public Affairs
FACT SHEET Office of Public Affairs Phone: 301-415-8200 Email: [email protected] Uranium Recovery Background The production of fuel for nuclear power plants starts with taking uranium ore from the ground and then purifying and processing it through a series of steps. Uranium recovery focuses on extracting natural uranium ore from the earth and concentrating (or milling) that ore. These recovery operations produce a product, called "yellowcake," which is then transported to a succession of fuel cycle facilities where the yellowcake is transformed into fuel for nuclear power reactors. In addition to yellowcake, uranium recovery operations generate waste products, called byproduct materials, that contain low levels of radioactivity. The NRC does not regulate uranium mining or mining exploration, but does have authority over milling of mined materials and in situ processes used to recover uranium, as well as mill tailings. Today’s conventional uranium mills and in situ recovery (ISR) facilities are operating safely and in a manner that is protective of the environment. The NRC regulates these facilities in close coordination with other Federal agencies and State and Tribal governments and provides technical support and guidance to those Agreement States that have authority over uranium recovery activities. Discussion The NRC becomes involved in uranium recovery operations when the ore is processed and physically or chemically altered. This happens either in a conventional, heap leach uranium mill, or ISR. For that reason, the NRC regulates ISR facilities as well as uranium mills and the disposal of liquid and solid wastes from uranium recovery operations (including mill tailings). -

Per-10 Basic Information Relating to Uranium
PER-10 1 BASIC INFORMATION RELATING TO URANIUM-ENRICHMENT CALCULATIONS AND FUEL REQUIREMENTS FOR NUCLEAR POWER REACTORS by K.T. Brown m 3 ATOMIC ENERGY BOARD Pelindaba PRETORIA Republic of South Africa February 1977 :::: : =:::""""""::::::;::: i:::""""""" :::::::::i:::::::::::::::H:::""»"""::::::::::::::::: BASIC INFORMATION RELATING TO URANIUM-ENRICHMENT CALCULATIONS AND FUEL REQUIREMENTS FOR NUCLEAR POWER REACTORS hy K.T. Brown POSTAL ADDRESS: Atomic Energy Board Private Bag X256 PRETORIA 0001 PELINDABA Fi-ln liai v 1977 ISBN U 86Ü6U 654 9 Pago Page SAMEVATTING 2 ABSTRACT 2 3. REACTOR FUEL REQUIREMENTS 5 1. INTRODUCTION 3 3.1 Reactor Types 5 2. URANIUM ENRICHMENT 3 3.1.1 Pressurised-water roactor 5 2.1 Definitions 3 3.1.2 Boiling-water reactor 5 2.1.1 Natural uranium 3 3.1.3 CANDU-PHW 6 2.1.2 Fissile 3 3.1.4 High-temperature gas-cooled reactor 6 2.1.3 Fertile 3 2.1.5 Liquid-metal-cooled fast breeder reactor ... .6 2.1.4 Enrichment 3 3.2 Nuclear Fuel Cycles 6 2.1.5 Product 3 3.3 Typical Fuel Requirements 6 2.1.6 Feed 3 3.3.1 Pressurised-wator reactor 7 2.1.7 Tails, or waste 3 3.3.2 Boiling-water reactor 8 2.1.8 Cascade 3 3.3.3 CANDU-PHW 9 2.1.9 Separative work 4 3.3.4 High-temperature gas-cooled reactor 9 2.1.10 Separative-work unit 4 3.3.5 Liquid-metal-cooled fast breeder reactor ... 10 2.2 Enrichment Parameters 4 3.3.6 Comparative data 10 2.3 Optimum Tails Assay 5 4. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Implementing Safeguards-By-Design at Natural Uranium Conversion Plants
NIS Office of Nuclear Safeguards and Security Safeguards-By-Design Facility Guidance Series (NGSI-SBD-002) August 2012 Implementing Safeguards-by-design at Natural Uranium Conversion Plants U.S. DEPARTMENT OF ENERG National Nuclear Security AdministrationY IMPLEMENTING SAFEGUARDS-BY-DESIGN AT NATURAL URANIUM CONVERSION PLANTS Lisa Loden John Begovich Date Published: July 2012 iii CONTENTS Page CONTENTS ......................................................................................................................................... IV 1. INTRODUCTION AND PURPOSE ................................................................................................. 1 2. KEY DEFINITIONS ......................................................................................................................... 2 3. SAFEGUARDS AT NUCPS ............................................................................................................. 7 3.1 SAFEGUARDS OBJECTIVES ................................................................................................. 7 3.2 TRADITIONAL AND INTEGRATED SAFEGUARDS ......................................................... 7 3.3 SAFEGUARDS RESPONSIBILITIES ..................................................................................... 8 3.3.1 STATE REGULATORY AUTHORITY RESPONSIBILITIES ..................................... 8 3.3.2 IAEA RESPONSIBILITIES ............................................................................................ 9 4. ELEMENTS OF FACILITY DESIGN THAT ARE RELEVANT -

FRANCIUM Element Symbol: Fr Atomic Number: 87
FRANCIUM Element Symbol: Fr Atomic Number: 87 An initiative of IYC 2011 brought to you by the RACI KAYE GREEN www.raci.org.au FRANCIUM Element symbol: Fr Atomic number: 87 Francium (previously known as eka-cesium and actinium K) is a radioactive metal and the second rarest naturally occurring element after Astatine. It is the least stable of the first 103 elements. Very little is known of the physical and chemical properties of Francium compared to other elements. Francium was discovered by Marguerite Perey of the Curie Institute in Paris, France in 1939. However, the existence of an element of atomic number 87 was predicted in the 1870s by Dmitri Mendeleev, creator of the first version of the periodic table, who presumed it would have chemical and physical properties similar to Cesium. Several research teams attempted to isolate this missing element, and there were at least four false claims of discovery during which it was named Russium (after the home country of soviet chemist D. K. Dobroserdov), Alkalinium (by English chemists Gerald J. K. Druce and Frederick H. Loring as the heaviest alkali metal), Virginium (after Virginia, home state of chemist Fred Allison), and Moldavium (by Horia Hulubei and Yvette Cauchois after Moldavia, the Romanian province where they conducted their work). Perey finally discovered Francium after purifying radioactive Actinium-227 from Lanthanum, and detecting particles decaying at low energy levels not previously identified. The new product exhibited chemical properties of an alkali metal (such as co-precipitating with Cesium salts), which led Perey to believe that it was element 87, caused by the alpha radioactive decay of Actinium-227. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2017 The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin Mark Alan Moore University of Tennessee Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation Moore, Mark Alan, "The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin. " PhD diss., University of Tennessee, 2017. https://trace.tennessee.edu/utk_graddiss/4848 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Mark Alan Moore entitled "The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemical Engineering. Robert Counce, Major Professor We have read this dissertation and recommend its acceptance: Paul Dalhaimer, Howard Hall, George Schweitzer, Jack Watson Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Mark Alan Moore December 2017 Copyright © 2017 by Mark A. -

Experimental Γ Ray Spectroscopy and Investigations of Environmental Radioactivity
Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity BY RANDOLPH S. PETERSON 216 α Po 84 10.64h. 212 Pb 1- 415 82 0- 239 β- 01- 0 60.6m 212 1+ 1630 Bi 2+ 1513 83 α β- 2+ 787 304ns 0+ 0 212 α Po 84 Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity Randolph S. Peterson Physics Department The University of the South Sewanee, Tennessee Published by Spectrum Techniques All Rights Reserved Copyright 1996 TABLE OF CONTENTS Page Introduction ....................................................................................................................4 Basic Gamma Spectroscopy 1. Energy Calibration ................................................................................................... 7 2. Gamma Spectra from Common Commercial Sources ........................................ 10 3. Detector Energy Resolution .................................................................................. 12 Interaction of Radiation with Matter 4. Compton Scattering............................................................................................... 14 5. Pair Production and Annihilation ........................................................................ 17 6. Absorption of Gammas by Materials ..................................................................... 19 7. X Rays ..................................................................................................................... 21 Radioactive Decay 8. Multichannel Scaling and Half-life ..................................................................... -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties.