The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

FRANCIUM Element Symbol: Fr Atomic Number: 87
FRANCIUM Element Symbol: Fr Atomic Number: 87 An initiative of IYC 2011 brought to you by the RACI KAYE GREEN www.raci.org.au FRANCIUM Element symbol: Fr Atomic number: 87 Francium (previously known as eka-cesium and actinium K) is a radioactive metal and the second rarest naturally occurring element after Astatine. It is the least stable of the first 103 elements. Very little is known of the physical and chemical properties of Francium compared to other elements. Francium was discovered by Marguerite Perey of the Curie Institute in Paris, France in 1939. However, the existence of an element of atomic number 87 was predicted in the 1870s by Dmitri Mendeleev, creator of the first version of the periodic table, who presumed it would have chemical and physical properties similar to Cesium. Several research teams attempted to isolate this missing element, and there were at least four false claims of discovery during which it was named Russium (after the home country of soviet chemist D. K. Dobroserdov), Alkalinium (by English chemists Gerald J. K. Druce and Frederick H. Loring as the heaviest alkali metal), Virginium (after Virginia, home state of chemist Fred Allison), and Moldavium (by Horia Hulubei and Yvette Cauchois after Moldavia, the Romanian province where they conducted their work). Perey finally discovered Francium after purifying radioactive Actinium-227 from Lanthanum, and detecting particles decaying at low energy levels not previously identified. The new product exhibited chemical properties of an alkali metal (such as co-precipitating with Cesium salts), which led Perey to believe that it was element 87, caused by the alpha radioactive decay of Actinium-227. -

BISMUTH LEAD ALLOY Synonyms BISMUTH ALLOY, 5805, HS CODE 78019900 ● NYRSTAR LEAD ALLOYS
1. IDENTIFICATION OF THE MATERIAL AND SUPPLIER 1.1 Product identifier Product name BISMUTH LEAD ALLOY Synonyms BISMUTH ALLOY, 5805, HS CODE 78019900 ● NYRSTAR LEAD ALLOYS 1.2 Uses and uses advised against Uses ALLOY Used for further refining of bismuth and lead metals. 1.3 Details of the supplier of the product Supplier name NYRSTAR PORT PIRIE Address PO Box 219, Port Pirie, SA, 5540, AUSTRALIA Telephone (08) 8638 1500 Fax (08) 8638 1583 Website http://www.nyrstar.com 1.4 Emergency telephone numbers Emergency (08) 8638 1500 2. HAZARDS IDENTIFICATION 2.1 Classification of the substance or mixture CLASSIFIED AS HAZARDOUS ACCORDING TO SAFE WORK AUSTRALIA CRITERIA GHS classifications Acute Toxicity: Oral: Category 4 Acute Toxicity: Inhalation: Category 4 Toxic to Reproduction: Category 1A Specific Target Organ Systemic Toxicity (Repeated Exposure): Category 2 2.2 GHS Label elements Signal word DANGER Pictograms Hazard statements H302 Harmful if swallowed. H332 Harmful if inhaled. H360 May damage fertility or the unborn child. H373 May cause damage to organs through prolonged or repeated exposure. Prevention statements P202 Do not handle until all safety precautions have been read and understood. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P264 Wash thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P271 Use only outdoors or in a well-ventilated area. P281 Use personal protective equipment as required. SDS Date: 08 Jun 2018 Page 1 of 7 Version No: 1.3 PRODUCT NAME BISMUTH LEAD ALLOY Response statements P304 + P340 IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing. -

Zinc-Bright®
Zinc-bright® ® ZINC-BRIGHT®, a zinc-bismuth-tin alloy for the right quantity of both Bismuth and Tin Zinc-bright performancerforman- Lead-free galvanizing, was developed by to stabilize your bath at the right concen- Boliden to meet the needs of our custom- trations in order to reduces surface defects ers in the general galvanising industry. and improves the overall aspect. The easy to use, ready-made alloy means no more mixing of different components, OUR PROMISE saving our customers time, effort and Boliden’s experienced technical support costs. drives the development and production process of the metal. Our Technical Con- In some countries and in some applica- sultant is ready to answer your questions hhkkkkhkhk tions, Lead is an unwanted metal. Bismuth with regards to your individual application 3: standard performance of traditional zinc > 3: better performance is a candidate to replace Lead in order to of the product. Through reliable deliver- < 3: worse performance reduce surface tension. Addition of Tin ies, high quality products and professional According standard: ISO 1461 further enables a good wettability of the customer service, including price informa- steel work in the liquid Zinc. By adding tion we want to be your supplier of choice. Bismuth and Tin, the physical properties of Did you know that … the zinc melt are improved. You can simply By becoming Boliden’s customer, we want DidBoliden’s strength in alloys comes from replace the zinc in your bath with Zinc- to help you add value to your customers innovation driven by tight environmental legislation in Scandinavia for metal bright® without any change to your produc- and work with you for a more sustainable production. -

Adverse Health Effects of Heavy Metals in Children
TRAINING FOR HEALTH CARE PROVIDERS [Date …Place …Event …Sponsor …Organizer] ADVERSE HEALTH EFFECTS OF HEAVY METALS IN CHILDREN Children's Health and the Environment WHO Training Package for the Health Sector World Health Organization www.who.int/ceh October 2011 1 <<NOTE TO USER: Please add details of the date, time, place and sponsorship of the meeting for which you are using this presentation in the space indicated.>> <<NOTE TO USER: This is a large set of slides from which the presenter should select the most relevant ones to use in a specific presentation. These slides cover many facets of the problem. Present only those slides that apply most directly to the local situation in the region. Please replace the examples, data, pictures and case studies with ones that are relevant to your situation.>> <<NOTE TO USER: This slide set discusses routes of exposure, adverse health effects and case studies from environmental exposure to heavy metals, other than lead and mercury, please go to the modules on lead and mercury for more information on those. Please refer to other modules (e.g. water, neurodevelopment, biomonitoring, environmental and developmental origins of disease) for complementary information>> Children and heavy metals LEARNING OBJECTIVES To define the spectrum of heavy metals (others than lead and mercury) with adverse effects on human health To describe the epidemiology of adverse effects of heavy metals (Arsenic, Cadmium, Copper and Thallium) in children To describe sources and routes of exposure of children to those heavy metals To understand the mechanism and illustrate the clinical effects of heavy metals’ toxicity To discuss the strategy of prevention of heavy metals’ adverse effects 2 The scope of this module is to provide an overview of the public health impact, adverse health effects, epidemiology, mechanism of action and prevention of heavy metals (other than lead and mercury) toxicity in children. -

The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Alkali Metal Bismuth(III) Chloride Double Salts
W&M ScholarWorks Arts & Sciences Articles Arts and Sciences 2016 Alkali metal bismuth(III) chloride double salts Andrew W. Kelly College of William and Mary, Dept Chem, Williamsburg, VA 23187 USA Robert D. Pike College of William and Mary, Dept Chem, Williamsburg, VA 23187 USA Aaron Nicholas Univ Maine, Dept Chem, Orono, ME 04469 USA; John C. Ahern Univ Maine, Dept Chem, Orono, ME 04469 USA; Howard H. Patterson Univ Maine, Dept Chem, Orono, ME 04469 USA; Follow this and additional works at: https://scholarworks.wm.edu/aspubs Recommended Citation Kelly, A. W., Nicholas, A., Ahern, J. C., Chan, B., Patterson, H. H., & Pike, R. D. (2016). Alkali metal bismuth (III) chloride double salts. Journal of Alloys and Compounds, 670, 337-345. This Article is brought to you for free and open access by the Arts and Sciences at W&M ScholarWorks. It has been accepted for inclusion in Arts & Sciences Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Alkali Metal Bismuth(III) Chloride Double Salts Andrew W. Kelly,a Aaron Nicholas,b John C. Ahern,b Benny Chan,c Howard H. Patterson,b and Robert D. Pikea* aDepartment of Chemistry, College of William and Mary, Williamsburg, VA 23187. bDepartment of Chemistry, University of Maine, Orono, ME 04469. cDepartment of Chemistry, College of New Jersey, Ewing, NJ 08628-0718. Corresponding Author: Robert D. Pike Department of Chemistry College of William and Mary Williamsburg, VA 23187-8795. telephone: 757-221-2555 FAX: 757-221-2715 email: rdpike@ wm.edu 1 © 2016. This manuscript version is made available under the Elsevier user license http://www.elsevier.com/open-access/userlicense/1.0/ Abstract: Evaporative co-crystallization of MCl (M = Na, K, Rb, Cs) with BiOCl in aqueous HCl produces double salts: MxBiyCl(x+3y)•zH2O. -

Periodic Table of the Elements Notes
Periodic Table of the Elements Notes Arrangement of the known elements based on atomic number and chemical and physical properties. Divided into three basic categories: Metals (left side of the table) Nonmetals (right side of the table) Metalloids (touching the zig zag line) Basic Organization by: Atomic structure Atomic number Chemical and Physical Properties Uses of the Periodic Table Useful in predicting: chemical behavior of the elements trends properties of the elements Atomic Structure Review: Atoms are made of protons, electrons, and neutrons. Elements are atoms of only one type. Elements are identified by the atomic number (# of protons in nucleus). Energy Levels Review: Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms Energy Levels & Valence Electrons Energy levels hold a specific amount of electrons: 1st level = up to 2 2nd level = up to 8 3rd level = up to 8 (first 18 elements only) The electrons in the outermost level are called valence electrons. Determine reactivity - how elements will react with others to form compounds Outermost level does not usually fill completely with electrons Using the Table to Identify Valence Electrons Elements are grouped into vertical columns because they have similar properties. These are called groups or families. Groups are numbered 1-18. Group numbers can help you determine the number of valence electrons: Group 1 has 1 valence electron. Group 2 has 2 valence electrons. Groups 3–12 are transition metals and have 1 or 2 valence electrons. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -
Protactinium
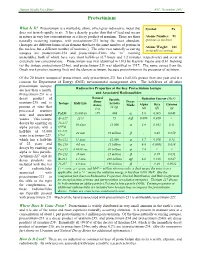
Human Health Fact Sheet ANL, November 2001 Protactinium What Is It? Protactinium is a malleable, shiny, silver-gray radioactive metal that Symbol: Pa does not tarnish rapidly in air. It has a density greater than that of lead and occurs in nature in very low concentrations as a decay product of uranium. There are three Atomic Number: 91 naturally occurring isotopes, with protactinium-231 being the most abundant. (protons in nucleus) (Isotopes are different forms of an element that have the same number of protons in Atomic Weight: 231 the nucleus but a different number of neutrons.) The other two naturally occurring (naturally occurring) isotopes are protactinium-234 and protactinium-234m (the “m” meaning metastable), both of which have very short half-lives (6.7 hours and 1.2 minutes, respectively) and occur in extremely low concentrations. Protactinium was first identified in 1913 by Kasimir Fajans and O.H. Gohring (as the isotope protactinium-234m), and protactinium-231 was identified in 1917. The name comes from the Greek work protos (meaning first) and the element actinium, because protactinium is the precursor of actinium. Of the 20 known isotopes of protactinium, only protactinium-231 has a half-life greater than one year and is a concern for Department of Energy (DOE) environmental management sites. The half-lives of all other protactinium isotopes Radioactive Properties of the Key Protactinium Isotope are less than a month. Protactinium-231 is a and Associated Radionuclides Natural decay product of Specific Radiation Energy (MeV) Abun- Decay uranium-235 and is Isotope Half-Life Activity dance Mode Alpha Beta Gamma present at sites that (Ci/g) (%) (α) (β) (γ) processed uranium α ores and associated Pa231 33,000 yr >99 .048 5.0 0.065 0.048 wastes. -

Attachment 2
Solving Important Problems with Thorium Reactors Kirk Sorensen Flibe Energy March 2, 2016 Our industrial civilization expects reliable, affordable energy. The energies that bind the atomic nucleus (nuclear energy) are approximately two million times greater than the energies that bind the atomic electrons (chemical energy). Three Nuclear Options Possible Nuclear Fuels Natural Thorium Natural Uranium 100% thorium-232 99.3% uranium-238 0.7% uranium-235 Only a small fraction of natural uranium is fissile. Most uranium and all thorium is "fertile" and can be converted to fissile material through neutron absorption. Reducing Long-Lived Waste I Today’s approach to nuclear energy consumes only a small amount of the energy content of uranium while producing "transuranic" nuclides that complicate long-term waste disposal. I Using thorium/U-233 in a liquid-fueled reactor can more nearly approach the ideal of a fission-product-only waste stream that reaches the same radioactivity as uranium ore in 300 years. Today’s nuclear fuel is fabricated with extraordinary precision, like a fine watch. But it is that precision that makes it difficult to recycle and to refabricate. A new approach is needed that is more versatile and less expensive. Fluoride salts are safe and versatile Chemically stable in air and water Unpressurized liquid with 1000◦C range of temperature LiF-BeF2 fluoride salt is an excellent carrier for uranium (UF4) nuclear fuel. Liquid fuels enhance safety options The reactor is equipped with a "freeze plug"—an open line where a frozen plug of salt is blocking the flow. The plug is kept frozen by an external cooling fan. -

(Lead-Tin-Bismuth-Cadmium) Having Low Melting Temperature
VAEC-AR 07--37 STUDY ON TECHNOLOGY FOR MANUFACTURING ALLOY (LEAD - TIN - BISMUTH - CADMIUM) HAVING LOW MELTING TEMPERATURE (≤ 800C) USED TO SHIELD RADIOACTIVE RAYS FOR TREATING CANCER Ngo Xuan Hung, Pham Duc Thai, Nguyen The Khanh Vu Quang Chat and Nguyen Huu Quyet Institute for Technology of Radioactive and Rare Elements, VAEC, Vietnam. ABSTRACT: Up to now, hospitals in Vietnam have mostly imported radioactive equipments from America, German, France, England to treat cancer. Accompany with those equipments, alloy, namely Cyroben having low melting temperature (≤ 800C) is used to cover patients’ good tissues in order to protect them against harmful rays and help radioactive rays get through the cast hole to kill cancer cells. This project is carried out for determining chemical compositions and melting temperatures of researched alloy to create alloy having low melting temperature (≤ 800C) to meet demand for treating cancer in Vietnam. Key words: Processing flow sheet, name element: Lead (Pb); Tin (Sn); Bismuth (Bi); Cadmium (Cd). INTRODUCTION The Cyroben alloy (Pb – Sn – Bi – Cd) used to cover the cancer has to meet 3 basic demands: - Meet technique characters of radioactive treating: can cover 92-95% of radioactive rays (compared with open radiation source) in order to keep those harmful rays out of patients’ good tissues as few as possible. - Have low melting temperature (≤ 800C), easy to melt and to cast in complex shapes. - Prevent workers, patients and doctors from harmful rays. EXPERIMENT RESULTS AND DISCUSSION After analyzing chemical compositions, measuring melting temperatures of imported alloy samples, we have received following technique parameters. We have used these parameters as well as studied foreign reference documents for carrying out experiments.