Mating Behavioral Function of Preoptic Galanin Neurons Is Shared Between Fish with Alternative Male Reproductive Tactics and Tetrapods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Article Evolutionary Dynamics of the OR Gene Repertoire in Teleost Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Article Evolutionary dynamics of the OR gene repertoire in teleost fishes: evidence of an association with changes in olfactory epithelium shape Maxime Policarpo1, Katherine E Bemis2, James C Tyler3, Cushla J Metcalfe4, Patrick Laurenti5, Jean-Christophe Sandoz1, Sylvie Rétaux6 and Didier Casane*,1,7 1 Université Paris-Saclay, CNRS, IRD, UMR Évolution, Génomes, Comportement et Écologie, 91198, Gif-sur-Yvette, France. 2 NOAA National Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. 3Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20560, U.S.A. 4 Independent Researcher, PO Box 21, Nambour QLD 4560, Australia. 5 Université de Paris, Laboratoire Interdisciplinaire des Energies de Demain, Paris, France 6 Université Paris-Saclay, CNRS, Institut des Neurosciences Paris-Saclay, 91190, Gif-sur- Yvette, France. 7 Université de Paris, UFR Sciences du Vivant, F-75013 Paris, France. * Corresponding author: e-mail: [email protected]. !1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Teleost fishes perceive their environment through a range of sensory modalities, among which olfaction often plays an important role. -

Integrative and Comparative Biology Integrative and Comparative Biology, Pp
Integrative and Comparative Biology Integrative and Comparative Biology, pp. 1–15 doi:10.1093/icb/icx053 Society for Integrative and Comparative Biology SYMPOSIUM Attention and Motivated Response to Simulated Male Advertisement Call Activates Forebrain Dopaminergic and Social Decision-Making Network Nuclei in Female Midshipman Fish Paul M. Forlano,*,†,‡,§,¶,1 Roshney R. Licorish,* Zachary N. Ghahramani,*,† Miky Timothy,* Melissa Ferrari,k William C. Palmer# and Joseph A. Sisneros#,** *Department of Biology, Brooklyn College, The City University of New York, Brooklyn, NY, USA; †Biology Subprogram in Ecology, Evolutionary Biology, and Behavior, The Graduate Center, City University of New York, New York, NY, USA; ‡Biology Subprogram in Neuroscience, The Graduate Center, City University of New York, New York, NY, USA; §Psychology Subprogram in Behavioral and Cognitive Neuroscience, The Graduate Center, City University of New York, New York, NY, USA; ¶Aquatic Research and Environmental Assessment Center, Brooklyn College, Brooklyn, NY, USA; kEdward R. Murrow High School, Brooklyn, NY, USA; #Department of Psychology, University of Washington, Seattle, WA, USA; **Virginia Bloedel Hearing Research Center, Seattle, WA, USA From the symposium “Integrating Cognitive, Motivational and Sensory Biases Underlying Acoustic and Multimodal Mate Choice” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 4–8, 2017 at New Orleans, Louisiana. 1E-mail: [email protected] Synopsis Little is known regarding the coordination of audition with decision-making and subsequent motor responses that initiate social behavior including mate localization during courtship. Using the midshipman fish model, we tested the hypothesis that the time spent by females attending and responding to the advertisement call is correlated with the activation of a specific subset of catecholaminergic (CA) and social decision-making network (SDM) nuclei underlying auditory- driven sexual motivation. -

Fish Feeding and Dynamics of Soft-Sediment Mollusc Populations in a Coral Reef Lagoon
MARINE ECOLOGY PROGRESS SERIES Published March 3 Mar. Ecol. Prog. Ser. Fish feeding and dynamics of soft-sediment mollusc populations in a coral reef lagoon G. P. Jones*, D. J. Ferrelle*,P. F. Sale*** School of Biological Sciences, University of Sydney, Sydney 2006, N.S.W., Australia ABSTRACT: Large coral reef fish were experimentally excluded from enclosed plots for 2 yr to examine their effect on the dynamics of soft sediment mollusc populations from areas in One Tree lagoon (Great Barrier Reef). Three teleost fish which feed on benthic molluscs. Lethrinus nebulosus, Diagramrna pictum and Pseudocaranx dentex, were common in the vicinity of the cages. Surveys of feeding scars in the sand indicated similar use of cage control and open control plots and effective exclusion by cages. The densities of 10 common species of prey were variable between locations and among times. Only 2 species exhibited an effect attributable to feeding by fish, and this was at one location only. The effect size was small relative to the spatial and temporal variation in numbers. The power of the test was sufficient to detect effects of fish on most species, had they occurred. A number of the molluscs exhibited annual cycles in abundance, with summer peaks due to an influx of juveniles but almost total loss of this cohort in winter. There was no evidence that predation altered the size-structure of these populations. While predation by fish is clearly intense, it does not have significant effects on the demo- graphy of these molluscs. The results cast doubt on the generality of the claim that predation is an important structuring agent in tropical communities. -

Exposure to Advertisement Calls of Reproductive Competitors Activates Vocal-Acoustic and Catecholaminergic Neurons in the Plainf
City University of New York (CUNY) CUNY Academic Works Publications and Research Brooklyn College 2013 Exposure to Advertisement Calls of Reproductive Competitors Activates Vocal-Acoustic and Catecholaminergic Neurons in the Plainfin Midshipman Fish, orichthysP notatus Christopher L. Petersen CUNY Brooklyn College Miky Timothy CUNY Brooklyn College D. Spencer Kim CUNY Brooklyn College Ashwin A. Bhandiwad University of Washington Robert A. Mohr University of Washington See next page for additional authors How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/bc_pubs/26 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Authors Christopher L. Petersen, Miky Timothy, D. Spencer Kim, Ashwin A. Bhandiwad, Robert A. Mohr, Joseph A. Sisneros, and Paul M. Forlano This article is available at CUNY Academic Works: https://academicworks.cuny.edu/bc_pubs/26 Exposure to Advertisement Calls of Reproductive Competitors Activates Vocal-Acoustic and Catecholaminergic Neurons in the Plainfin Midshipman Fish, Porichthys notatus Christopher L. Petersen1, Miky Timothy1, D. Spencer Kim1, Ashwin A. Bhandiwad2, Robert A. Mohr2, Joseph A. Sisneros2,3, Paul M. Forlano1,4,5* 1 Department of Biology, Brooklyn College, City University of New York, Brooklyn, New York, United States of America, 2 Department of Psychology, University of Washington, Seattle, Washington, United States -

Transposable Elements and Teleost Migratory Behaviour
International Journal of Molecular Sciences Article Transposable Elements and Teleost Migratory Behaviour Elisa Carotti 1,†, Federica Carducci 1,†, Adriana Canapa 1, Marco Barucca 1,* , Samuele Greco 2 , Marco Gerdol 2 and Maria Assunta Biscotti 1 1 Department of Life and Environmental Sciences, Polytechnic University of Marche, Via Brecce Bianche, 60131 Ancona, Italy; [email protected] (E.C.); [email protected] (F.C.); [email protected] (A.C.); [email protected] (M.A.B.) 2 Department of Life Sciences, University of Trieste, Via L. Giorgieri, 5-34127 Trieste, Italy; [email protected] (S.G.); [email protected] (M.G.) * Correspondence: [email protected] † Equal contribution. Abstract: Transposable elements (TEs) represent a considerable fraction of eukaryotic genomes, thereby contributing to genome size, chromosomal rearrangements, and to the generation of new coding genes or regulatory elements. An increasing number of works have reported a link between the genomic abundance of TEs and the adaptation to specific environmental conditions. Diadromy represents a fascinating feature of fish, protagonists of migratory routes between marine and fresh- water for reproduction. In this work, we investigated the genomes of 24 fish species, including 15 teleosts with a migratory behaviour. The expected higher relative abundance of DNA transposons in ray-finned fish compared with the other fish groups was not confirmed by the analysis of the dataset considered. The relative contribution of different TE types in migratory ray-finned species did not show clear differences between oceanodromous and potamodromous fish. On the contrary, a remarkable relationship between migratory behaviour and the quantitative difference reported for short interspersed nuclear (retro)elements (SINEs) emerged from the comparison between anadro- mous and catadromous species, independently from their phylogenetic position. -

Reef-Associated Fishes Have More Maneuverable Body Shapes at a Macroevolutionary Scale
Coral Reefs https://doi.org/10.1007/s00338-020-01976-w REPORT Reef-associated fishes have more maneuverable body shapes at a macroevolutionary scale 1 1 2 2 Olivier Larouche • Bailey Benton • Katherine A. Corn • Sarah T. Friedman • 1 1 1 2 Dominique Gross • Mikayla Iwan • Brian Kessler • Christopher M. Martinez • 1 1 2 1 Sierra Rodriguez • Hannah Whelpley • Peter C. Wainwright • Samantha A. Price Received: 25 March 2020 / Accepted: 9 July 2020 Ó Springer-Verlag GmbH Germany, part of Springer Nature 2020 Abstract Marine habitats vary widely in structure, from with narrower and shallower caudal peduncles. Despite the incredibly complex coral reefs to simpler deep water and numerous evolutionary forces that may influence body open ocean habitats. Hydromechanical models of swim- shapes on a broad macroevolutionary scale, our results ming kinematics and microevolutionary studies suggest reveal differences in body shapes between reef-associated that these habitats select for different body shape charac- and non-reef species that are consistent with hydrome- teristics. Fishes living in simple habitats are predicted to chanical models of swimming kinematics as well as with experience selection for energy-efficient sustained swim- microevolutionary patterns. ming, which can be achieved by fusiform body shapes. In contrast, fishes living in complex habitats are predicted to Keywords Habitat structural complexity Á Fish body be under selection for maneuverability, which can be shapes Á Fish swimming Á Macroevolution Á Morphological enhanced by deep-bodied and laterally compressed forms. diversity To look for a signature of these processes at a broad macroevolutionary scale, we quantified the body shapes of 3322 species of marine teleostean fishes using a series of Introduction linear measurements. -

KNABE's Dt-Posit $100 Required at Time of Sale
SUMMER RESORTS. SUMMER RESORTS. EDUCAONAL AUCTION SALES. AUCTION SALES. WAR FOR SALE-HOUSES. IR I WITH THE BOERS Iali$ SllE ci c i cN m'ItETEI) STriwET NEAR The Atlantio CIty Oee of The cArW wAT. N. J. OUT OF WHINGTON. -rM AWr RUO N. FUTURE DAYS. ; I.tlNI; 1IFFiCE. li4M11 ItRICK. Evening Star is located at 1308-18 N.1 52.:4to .\l.'ittS$ --EN'XCH.LIENT fP- THE BREXTGN. GAY HIL SCHOOL FOR.GIGRIM CHARLOTrE DUNCANSON BROS., AUCTIONEERS. JAMES W. RATCLIFFE, AUCTIONEER. Atlantie avenue, where any intforsa- season. Near the beach. St. county, Er.E I' clllTi N IT Y.- ST.\1it cFlit'iE ccu22-if ein Twelfth Large pl. Hall. Mary's 4md. T. BRIS- Now Regrd an a British tion concerning advertising, eta., ax=s ad man parlor. Terms moderate. tang- COE, Principol. , , aul9-2w TRUSTEES' SALE OF TWO FIVE-ROOM FRAME TRUSTEES' SALE OF A TWO-STORY BRICK Oertainty by } cct s.1 IF 'ct' li.FVE $ _._tt " lASl AND be obtained. The rates are the same distance 42. Mrs. J. A. MYERS. HOUSES ON SECOND STREET NEAR M AND FRAME STORE AND DWELLINO. AND in lh!,l .iil net 'phone :t a b:at pay *12. tmithly an Home 01lee. myl-m,w&0-4M ACAD3WY. Rockvtlle, MW, for BoyS. Its ils STRELT SOUTHEAST. A TWO-STORY FRAME HOUSE. KNOWN Ad Foreign Offic3. on ~ ~ ~ I Uh, '.Iv. le hwYou Ih..osse charged at the have done well at the Unive. of..V., Corne. On TUESDAY THE TWENTY-SECOND DAY NOS. 314 i.ND 316 EAST CAPITOL STREET. -

Rhodopsin Gene Evolution in Early Teleost Fishes
RESEARCH ARTICLE Rhodopsin gene evolution in early teleost fishes 1 2 1 Jhen-Nien Chen , Sarah Samadi , Wei-Jen ChenID * 1 Institute of Oceanography, National Taiwan University, Taipei, Taiwan, 2 Institute de SysteÂmatique, E volution, Biodiversite (ISYEB), MuseÂum National d'Histoire Naturelle±CNRS, Sorbonne UniversiteÂ, EPHE, Paris, France * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Rhodopsin mediates an essential step in image capture and is tightly associated with visual adaptations of aquatic organisms, especially species that live in dim light environments (e.g., the deep sea). The rh1 gene encoding rhodopsin was formerly considered a single- copy gene in genomes of vertebrates, but increasing exceptional cases have been found in teleost fish species. The main objective of this study was to determine to what extent the OPEN ACCESS visual adaptation of teleosts might have been shaped by the duplication and loss of rh1 Citation: Chen J-N, Samadi S, Chen W-J (2018) genes. For that purpose, homologous rh1/rh1-like sequences in genomes of ray-finned Rhodopsin gene evolution in early teleost fishes. PLoS ONE 13(11): e0206918. https://doi.org/ fishes from a wide taxonomic range were explored using a PCR-based method, data mining 10.1371/journal.pone.0206918 of public genetic/genomic databases, and subsequent phylogenomic analyses of the Editor: Michael Schubert, Laboratoire de Biologie retrieved sequences. We show that a second copy of the fish-specific intron-less rh1 is pres- du DeÂveloppement de Villefranche-sur-Mer, ent in the genomes of most anguillids (Elopomorpha), Hiodon alosoides (Osteoglossomor- FRANCE pha), and several clupeocephalan lineages. -

The Plainfin Midshipman's Soundscape at Two Sites Around Vancouver Island, British Columbia
Vol. 603: 189–200, 2018 MARINE ECOLOGY PROGRESS SERIES Published September 17 https://doi.org/10.3354/meps12730 Mar Ecol Prog Ser The plainfin midshipman’s soundscape at two sites around Vancouver Island, British Columbia William D. Halliday1,2,*, Matthew K. Pine1,2, Aneesh P. H. Bose3,4, Sigal Balshine3, Francis Juanes2 1Wildlife Conservation Society Canada, Whitehorse, Yukon Y1A 0E9, Canada 2Department of Biology, University of Victoria, Victoria, British Columbia V8P 5C2, Canada 3Department of Psychology, Neuroscience & Behaviour, McMaster University, Hamilton, Ontario L8S 4K1, Canada 4Present address: Karl-Franzens-Universität Graz, Institute of Biology, 8010 Graz, Austria ABSTRACT: The soundscape is an integral habitat component for acoustically sensitive animals. In marine environments, noise pollution from anthropogenic activities is pervasive, potentially leading to negative consequences for marine animals. To understand the impacts of noise pollu- tion, one must first understand the soundscape in which these animals live. Using autonomous passive acoustic recorders, we examined the soundscape of plainfin midshipman fish Porichthys notatus at 2 breeding sites around Vancouver Island, Canada. Plainfin midshipman humming was recorded every night for the 4 wk long recording period; it was a main driver of sound pressure levels, adding more than 6 and 17 dB on average (SE ± 0.8) at each site in the 80 Hz octave band. The fundamental frequency of the hum was temperature-dependent and varied between 76 and 111 Hz. At one site (Ladysmith Inlet), sound pressure level was consistently higher than at the other site (Brentwood Bay), and these differences appeared to be related to anthropogenic noise rather than to plainfin midshipman humming. -

A Single-Neuron: Current Trends and Future Prospects
cells Review A Single-Neuron: Current Trends and Future Prospects Pallavi Gupta 1, Nandhini Balasubramaniam 1, Hwan-You Chang 2, Fan-Gang Tseng 3 and Tuhin Subhra Santra 1,* 1 Department of Engineering Design, Indian Institute of Technology Madras, Tamil Nadu 600036, India; [email protected] (P.G.); [email protected] (N.B.) 2 Department of Medical Science, National Tsing Hua University, Hsinchu 30013, Taiwan; [email protected] 3 Department of Engineering and System Science, National Tsing Hua University, Hsinchu 30013, Taiwan; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +91-044-2257-4747 Received: 29 April 2020; Accepted: 19 June 2020; Published: 23 June 2020 Abstract: The brain is an intricate network with complex organizational principles facilitating a concerted communication between single-neurons, distinct neuron populations, and remote brain areas. The communication, technically referred to as connectivity, between single-neurons, is the center of many investigations aimed at elucidating pathophysiology, anatomical differences, and structural and functional features. In comparison with bulk analysis, single-neuron analysis can provide precise information about neurons or even sub-neuron level electrophysiology, anatomical differences, pathophysiology, structural and functional features, in addition to their communications with other neurons, and can promote essential information to understand the brain and its activity. This review highlights various single-neuron models and their behaviors, followed by different analysis methods. Again, to elucidate cellular dynamics in terms of electrophysiology at the single-neuron level, we emphasize in detail the role of single-neuron mapping and electrophysiological recording. We also elaborate on the recent development of single-neuron isolation, manipulation, and therapeutic progress using advanced micro/nanofluidic devices, as well as microinjection, electroporation, microelectrode array, optical transfection, optogenetic techniques. -
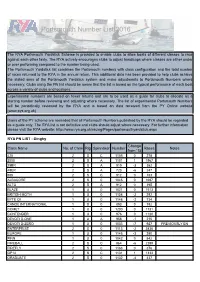
Portsmouth Number List 2016
Portsmouth Number List 2016 The RYA Portsmouth Yardstick Scheme is provided to enable clubs to allow boats of different classes to race against each other fairly. The RYA actively encourages clubs to adjust handicaps where classes are either under or over performing compared to the number being used. The Portsmouth Yardstick list combines the Portsmouth numbers with class configuration and the total number of races returned to the RYA in the annual return. This additional data has been provided to help clubs achieve the stated aims of the Portsmouth Yardstick system and make adjustments to Portsmouth Numbers where necessary. Clubs using the PN list should be aware that the list is based on the typical performance of each boat across a variety of clubs and locations. Experimental numbers are based on fewer returns and are to be used as a guide for clubs to allocate as a starting number before reviewing and adjusting where necessary. The list of experimental Portsmouth Numbers will be periodically reviewed by the RYA and is based on data received from the PY Online website (www.pys.org.uk). Users of the PY scheme are reminded that all Portsmouth Numbers published by the RYA should be regarded as a guide only. The RYA list is not definitive and clubs should adjust where necessary. For further information please visit the RYA website: http://www.rya.org.uk/racing/Pages/portsmouthyardstick.aspx RYA PN LIST - Dinghy Change Class Name No. of Crew Rig Spinnaker Number Races Notes from '15 420 2 S C 1105 0 278 2000 2 S A 1101 1 1967 29ER 2 S A -
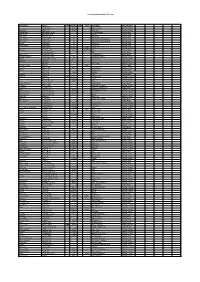
C:\Boatlists\Boatlistdraft-2021.Xlsx Boat Name Owner Prefix Sail No
C:\BoatLists\boatlistdraft-2021.xlsx Boat Name Owner Prefix Sail No. Suffix Hull Boat Type Classification Abraham C 2821 RS Feva XL Sailing Dinghy Dunikolu Adams R 10127 Wayfarer Sailing Dinghy Masie Mary Adlington CPLM 18ft motorboat Motor Boat Isla Rose Adlington JPN Tosher Sailing Boat Demelza Andrew JA 28 Heard 28 Sailing Boat Helen Mary Andrew KC 11 Falmouth Working Boat Sailing Boat Mary Ann Andrew KC 25 Falmouth Working Boat Sailing Boat Verity Andrew N 20 Sunbeam Sailing Boat West Wind Andrew N 21 Tosher 20 Sailing Boat Andrews K 208210 white Laser 4.7 Sailing Dinghy Hermes Armitage AC 70 dark blue Ajax Sailing Boat Armytage CD RIB Motor Boat Alice Rose Ashworth TGH Cockwell's 38 Motor Boat Maggie O'Nare Ashworth TGH 10 Cornish Crabber Sailing Cruiser OMG Ashworth* C & G 221 Laser Pico Sailing Dinghy Alcazar Bailey C Motor Boat Bailey C RS Fevqa Sailing Dinghy Dither of Dart Bailey T white Motor Sailer Coconi Barker CB 6000 Contessa 32 Sailing Cruiser Diana Barker G Rustler 24 Sailing Boat Barker G 1140 RS200 Sailing Dinghy Gemini Barnes E RIB Motor Boat Pelorus Barnes E GBR 3731L Arcona 380 Sailing Cruiser Barnes E 177817 Laser Sailing Dinghy Barnes F & W 1906 29er Sailing Dinghy Lady of Linhay Barnes MJ Catamaran Motor Boat Triumph Barnes MJ Westerly Centaur Sailing Cruiser Longhaul Barstow OG Orkney Longliner 16 Motor Boat Barö Barstow OG 2630 Marieholm IF-Boat Sailing Cruiser Rinse & Spin Bateman MCW 5919 Laser Pico Sailing Dinghy Why Hurry Batty-Smith JR 9312 Mirror Sailing Dinghy Natasha Baylis M Sadler 26 Sailing Cruiser