Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Male Reproductive System
Management of Men’s Reproductive 3 Health Problems Men’s Reproductive Health Curriculum Management of Men’s Reproductive 3 Health Problems © 2003 EngenderHealth. All rights reserved. 440 Ninth Avenue New York, NY 10001 U.S.A. Telephone: 212-561-8000 Fax: 212-561-8067 e-mail: [email protected] www.engenderhealth.org This publication was made possible, in part, through support provided by the Office of Population, U.S. Agency for International Development (USAID), under the terms of cooperative agreement HRN-A-00-98-00042-00. The opinions expressed herein are those of the publisher and do not necessarily reflect the views of USAID. Cover design: Virginia Taddoni ISBN 1-885063-45-8 Printed in the United States of America. Printed on recycled paper. Library of Congress Cataloging-in-Publication Data Men’s reproductive health curriculum : management of men’s reproductive health problems. p. ; cm. Companion v. to: Introduction to men’s reproductive health services, and: Counseling and communicating with men. Includes bibliographical references. ISBN 1-885063-45-8 1. Andrology. 2. Human reproduction. 3. Generative organs, Male--Diseases--Treatment. I. EngenderHealth (Firm) II. Counseling and communicating with men. III. Title: Introduction to men’s reproductive health services. [DNLM: 1. Genital Diseases, Male. 2. Physical Examination--methods. 3. Reproductive Health Services. WJ 700 M5483 2003] QP253.M465 2003 616.6’5--dc22 2003063056 Contents Acknowledgments v Introduction vii 1 Disorders of the Male Reproductive System 1.1 The Male -
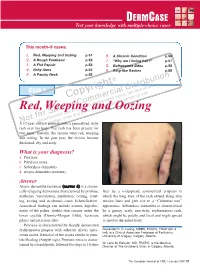
Red, Weeping and Oozing P.51 6
DERM CASE Test your knowledge with multiple-choice cases This month–9 cases: 1. Red, Weeping and Oozing p.51 6. A Chronic Condition p.56 2. A Rough Forehead p.52 7. “Why am I losing hair?” p.57 3. A Flat Papule p.53 8. Bothersome Bites p.58 4. Itchy Arms p.54 9. Ring-like Rashes p.59 5. A Patchy Neck p.55 on © buti t ri , h ist oad rig D wnl Case 1 y al n do p ci ca use o er sers nal C m d u rso m rise r pe o utho y fo C d. A cop or bite ngle Red, Weleepirnohig a sind Oozing a se p rint r S ed u nd p o oris w a t f uth , vie o Una lay AN12-year-old boy dpriesspents with a generalized, itchy rash over his body. The rash has been present for two years. Initially, the lesions were red, weeping and oozing. In the past year, the lesions became thickened, dry and scaly. What is your diagnosis? a. Psoriasis b. Pityriasis rosea c. Seborrheic dermatitis d. Atopic dermatitis (eczema) Answer Atopic dermatitis (eczema) (answer d) is a chroni - cally relapsing dermatosis characterized by pruritus, later by a widespread, symmetrical eruption in erythema, vesiculation, papulation, oozing, crust - which the long axes of the rash extend along skin ing, scaling and, in chronic cases, lichenification. tension lines and give rise to a “Christmas tree” Associated findings can include xerosis, hyperlin - appearance. Seborrheic dermatitis is characterized earity of the palms, double skin creases under the by a greasy, scaly, non-itchy, erythematous rash, lower eyelids (Dennie-Morgan folds), keratosis which might be patchy and focal and might spread pilaris and pityriasis alba. -

Activation of Flat Warts (Verrucae Planae) on the Q-Switched Laser-Assisted Tattoo Removal Site
CR(Case Report) Med Laser 2014;3(2):84-86 pISSN 2287-8300ㆍeISSN 2288-0224 Activation of Flat Warts (Verrucae Planae) on the Q-Switched Laser-Assisted Tattoo Removal Site Nark-kyoung Rho Flat warts, or verrucae planae, are a common cutaneous infection, which tends to be multiple and grouped. They are more often found on sun- exposed areas such as the face, neck, and extremities, and sometimes Leaders Aesthetic Laser and Cosmetic Surgery develop at the sites of skin trauma. The author reports on a case of flat Center, Seoul, Korea warts which developed on the Q-switched laser tattoo removal site. Histologic findings confirmed the diagnosis of verruca plana. The author suggests that activation of the human papillomavirus should be included in the possible complications of the laser-assisted tattoo removal procedure. Key words Human papillomavirus; Q-switched lasers; Tattooing; Warts Received November 28, 2014 Revised December 3, 2014 Accepted December 4, 2014 Correspondence Nark-kyoung Rho Leaders Aesthetic Laser and Cosmetic Surgery Center, THE CLASSIC500, 90, Neungdong-ro, Gwangjin-gu, Seoul 143-854, Korea Tel: +82-2-444-7585 Fax: +82-2-444-7535 E-mail: [email protected] C Korean Society for Laser Medicine and Surgery CC This is an open access article distributed under the terms of the Creative Commons Attribution Non- Commercial License (http://creativecommons.org/ licenses/by-nc/3.0) which permits unrestricted non- commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. 84 Medical Lasers; Engineering, Basic Research, and Clinical Application Warts on the Laser Tattoo Removal Site Nark-kyoung Rho Case Report INTRODUCTION mm spot size, and fluence of 12 J/cm2. -

Human Papillomavirus Infection in Child G
The Open Dermatology Journal, 2009, 3, 111-116 111 Open Access Human Papillomavirus Infection in Child G. Fabbrocini*, S. Cacciapuoti and G. Monfrecola Department of Systematic Pathology, Section of Dermatology, University of Naples Federico II, Naples, Italy Abstract: Human papilloma viruses (HPV) have been identified as the cause of cutaneous and genital warts. Furthermore, HPV DNA can also be detected in certain malignant epithelial tumors such as cervical carcinoma and cutaneous squamous cell cancer. HPV infections in children, particularly when occurring as condylomata acuminata, present a difficult and often puzzling problem. The modes of viral transmission in child remain controversial, including perinatal transmission, auto- and hetero-inoculation, sexual abuse, and, possibly, indirect transmission via fomites. The treatment of warts and condylomata acuminate in child poses a therapeutic challenge for physicians. No single therapy has been proven effective at achieving complete remission in every patient. As a result, many different approaches to therapy exist. The proper approach to the management of warts depends on the age of the patient, location, size, extent, and type of wart, and duration of lesions. Each treatment decision should be made on a case-by-case basis according to the experience of the physician, patient preference, and the application of evidence-based medicine. In order to modify HPV epidemiology, HPV prophylactic vaccine has been recently proposed for children. The purpose of this review is to update the reader with the latest information on the HPV and its therapeutics in children. Keywords: HPV, child, perinatal transmission, sexual abuse. INTRODUCTION because they have not yet been propagated in tissue culture. -

Gr up SM Skin In
SMGr up Skin in HIV Title: Skin in HIV Editor: Leelavathy Budamakuntla Published by SM Online Publishers LLC Copyright © 2015 SM Online Publishers LLC ISBN: 978-0-9962745-2-4 All book chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of the publication. Upon publication of the eBook, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work, identifying the original source. Statements and opinions expressed in the book are these of the individual contributors and not necessarily those of the editors or publisher. No responsibility is accepted for the accuracy of information contained in the published chapters. The publisher assumes no responsibility for any damage or injury to persons or property arising out of the use of any materials, instructions, methods or ideas contained in the book. First published April, 2015 Online Edition available at http://www.smgebooks.com For reprints, please contact us at [email protected] Skin in HIV | www.smgebooks.com 1 SMGr up Cutaneous Infections in HIV Disease Eswari L and Merin Paul P Department of Dermatology, STD and Leprosy, Bangalore Medical College and Research Institute, India. *Corresponding author: Leelavathy B, Department of Dermatology, STD and Leprosy, Bow- ring and Lady Curzon Hospital, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India, Email: [email protected] Published Date: April 15, 2015 INTRODUCTION Diagnosing and managing cutaneous infections in a HIV positive patient is a formidable and challenging task. -

PATHOLOGY of HIV/AIDS 32Nd Edition
PATHOLOGY OF HIV/AIDS 32nd Edition by Edward C. Klatt, MD Professor of Pathology Department of Biomedical Sciences Mercer University School of Medicine Savannah, Georgia, USA July 20, 2021 Copyright © by Edward C. Klatt, MD All rights reserved worldwide 2 DEDICATION To persons living with HIV/AIDS past, present, and future who provide the knowledge, to researchers who utilize the knowledge, to health care workers who apply the knowledge, and to public officials who do their best to promote the health of their citizens with the knowledge of the biology, pathophysiology, treatment, and prevention of HIV/AIDS. 3 TABLE OF CONTENTS 3.................................................................................................................................2 CHAPTER 1 - BIOLOGY AND PATHOGENESIS OF HIV INFECTION.....6 INTRODUCTION......................................................................................................................6 BIOLOGY OF HUMAN IMMUNODEFICIENCY VIRUS................................................10 HUMAN IMMUNODEFICIENCY VIRUS SUBTYPES.....................................................29 OTHER HUMAN RETROVIRUSES....................................................................................31 EPIDEMIOLOGY OF HIV/AIDS.........................................................................................36 RISK GROUPS FOR HUMAN IMMUNODEFICIENCY VIRUS INFECTION.............46 NATURAL HISTORY OF HIV INFECTION......................................................................47 PROGRESSION OF HIV -

DETECTION and MOLECULAR CHARACTERIZATION of MANATEE PAPILLOMAVIRUS in CUTANEOUS LESIONS of the FLORIDA MANATEE (Trichechus Manatus Latirostris)
DETECTION AND MOLECULAR CHARACTERIZATION OF MANATEE PAPILLOMAVIRUS IN CUTANEOUS LESIONS OF THE FLORIDA MANATEE (Trichechus manatus latirostris) By REBECCA ANN WOODRUFF A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2005 Copyright 2005 by Rebecca Ann Woodruff This document is dedicated to the graduate students of the University of Florida. ACKNOWLEDGMENT I would like to extend my greatest appreciation and thanks to my mentor, Dr. Carlos Romero, for allowing me to join his laboratory and enjoy many opportunities that I would not have been able to elsewhere. I would also like to thank my committee members, Dr. Peter McGuire and Dr. Ayalew Mergia, for their time, assistance, and suggestions. I would like to thank Dr. Ellis Greiner for first introducing me to the pathobiology graduate program at the University of Florida. I extend great thanks to Bob Bonde, not only for obtaining all of the manatee samples used in this study, but also for providing his expertise and knowledge of manatees. This project was funded by the Florida Fish and Wildlife Commission through the Marine Mammal Animal Health Program of the College of Veterinary Medicine at the University of Florida, and would not have been possible without the U. S. Geological Survey (USGS) Department of the Interior, contributions of collaborators affiliated with numerous zoological parks, and those involved with manatee capture release studies. I would also like to thank my fellow lab-mates, Alexa Bracht, Kara Smolarek-Benson, Shasta McClenahan, and Rebecca Grant, for their much valued friendships. -

Syphilis and Other Treponematoses (Clinical and Treatment)
Br J Vener Dis: first published as 10.1136/sti.60.5.352 on 1 October 1984. Downloaded from Br J Vener Dis 1984; 60:352-6 Abstracts These selected abstracts and titlesfrom the world literature are arranged in thefollowing sections: Syphilis and other treponematoses (clinical and Trichomoniasis treatment; serology and biologicalfalsepositive Candidosis phenomenon; pathology and experimental) Genital herpes Gonorrhoea (clinical; microbiology; treatment) Other sexually transmitted diseases Chlamydial infections Public health and social aspects Non-specific genital infection Miscellaneous Reiter's disease and midline lumber pain that was worse and a prolonged transit time. The illness Syphilis and other when lying supine. Five months before subsided without treatment after four days. treponematoses (clinical and admission she had a non-itchy dry rash on Similar episodes occurred during the treatment) her trunk and extremities, with lesions following year at four to eight week between five and 15 mm in diameter. intervals. Clinical findings on admission were of After one year he was found to have Penicillin treatment of early syphilis II diminished position sense in the lower perianal and mouth ulcers, hair loss, JF MAHONEY, RC ARNOLD, BL STERNER, ET AL extremities and increased pinprick sense hearing loss with tinnitus, and blurred (Staten Island, New York, USA). JAMA below the T5 dermatone. Muscle tone in the vision with papilloedema. A Venereal 1984; 251:2005-10. legs was also diminished. She had an Disease Research Laboratory (VDRL) test extensor plantar response on the left. The gave a positive result at a titre of 1/64. His Penicillin and early syphilis cranial nerves and arms gave normal results cerebrospinal fluid (CSF) showed: cells HL ARNOLD (Honolulu, Hawaii, USA). -

EXPERIMENTAL INOCULATION of HUMANS with ECTODERMOTROPIC VIRUSES* HERBERT GOLDSCHMIDT, Ml)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector EXPERIMENTAL INOCULATION OF HUMANS WITH ECTODERMOTROPIC VIRUSES* HERBERT GOLDSCHMIDT, Ml). AND ALBERT M. KLIGMAN, M.D., Pu.D. Students of human infectious disease have a Vaccinia much greater opportunity to gain an under- To test the hypothesis that freshly regenerated standing of pathogenesis when the disease can beepidermis would promote virus infection, we did experimentally reproduced in man at will. In thisa pilot study with vaccinia in view of the ease of paper we shall describe our efforts to promote thevaccination and the definite knowledge of its inoculation of human skin with the followingcourse. The vaccinations were carried out in the cctodcrmotropic viruses: the wart virus (verruca usual way (multiple puncture technic), using 106 vulgaris and condyloma acuminatum), vaccinia,adult subjects, all of whom had been previously molluscum contagiosum, herpes simplex andvaccinated. The anticipated immune response herpes zoster. A review of previous attempts iswas observed in every instance. In all, 312 inocu- appended. lations were made, one forearm site serving as METHODS the control with the corresponding site on the opposite forearm having been prepared either With the exception of the vaccinia virus, it isby dermabrasion or by cantharidin. The inocula- not at all easy to inoculate humans successfullytions were performed at intervals varying from 1 with the above mentioned viruses. "Takes" areto 18 weeks after preparation. As a standard of infrequent and erratic. Knowing that virusescomparison, the diameters of the lesions (exclu- multiply best in embryonic tissue, the assumptionsive of simple erythema) were measured on the was made that regenerating epidermal cells afterthird day post vaccination. -

Human RHOH Deficiency Causes T Cell Defects and Susceptibility to EV-HPV Infections
Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections Amandine Crequer, … , Jean-Laurent Casanova, Emmanuelle Jouanguy J Clin Invest. 2012;122(9):3239-3247. https://doi.org/10.1172/JCI62949. Research Article Immunology Epidermodysplasia verruciformis (EV) is a rare genetic disorder characterized by increased susceptibility to specific human papillomaviruses, the betapapillomaviruses. These EV-HPVs cause warts and increase the risk of skin carcinomas in otherwise healthy individuals. Inactivating mutations in epidermodysplasia verruciformis 1 (EVER1) or EVER2 have been identified in most, but not all, patients with autosomal recessive EV. We found that 2 young adult siblings presenting with T cell deficiency and various infectious diseases, including persistent EV-HPV infections, were homozygous for a mutation creating a stop codon in the ras homolog gene family member H (RHOH) gene. RHOH encodes an atypical Rho GTPase expressed predominantly in hematopoietic cells. Patients’ circulating T cells contained predominantly effector memory T cells, which displayed impaired TCR signaling. Additionally, very few circulating T cells expressed the β7 integrin subunit, which homes T cells to specific tissues. Similarly,R hoh-null mice exhibited a severe overall T cell defect and abnormally small numbers of circulating β7-positive cells. Expression of the WT, but not of the mutated RHOH, allele in Rhoh–/– hematopoietic stem cells corrected the T cell lymphopenia in mice after bone marrow transplantation. We conclude that RHOH deficiency leads to T cell defects and persistent EV-HPV infections, suggesting that T cells play a role in the pathogenesis of chronic EV-HPV infections. Find the latest version: https://jci.me/62949/pdf Research article Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections Amandine Crequer,1,2 Anja Troeger,3 Etienne Patin,4 Cindy S. -

Osmotically Active Hydrogels of Acrylics: Characterization And
LASER TREATMENT OF SCARS IN DARK SKIN AND WARTS Ph.D. dissertation Department of Dermatology and Allergology Albert Szent-Györgyi Medical Center University of Szeged 2011 Szeged, Hungary LASER TREATMENT OF SCARS IN DARK SKIN AND WARTS Ph.D. dissertation Ashraf Mohamed Abdel Magid Badawi M.B.B.Ch, M.Sc. (Dermatology and Andrology) Lecturer of Dermatology Supervisor: Lajos Kemény M.D., Ph.D., D.Sc. Department of Dermatology and Allergology Albert Szent-Györgyi Medical Center University of Szeged 2011 Szeged, Hungary Table of Contents Page LIST OF PUBLICATIONS 3 List of articles related to the subject of the dissertation 3 List of articles indirectly related to the subject of the dissertation 3 1. LIST OF ABBREVIATIONS 4 2. INTRODUCTION 5 2.1. Background 5 2.1.1. Laser treatment of scars 5 2.1.2. Laser treatment of warts 6 2.2. Aims 8 3. PATIENTS AND METHODS 9 3.1. Treatments of scars 9 3.1.1. Scars treatment in dark skin 9 3.1.2. Post chickenpox scars treatment 12 3.2. Treatment of warts 14 3.2.1. Treatments of genital warts 14 3.2.2. Treatments of plane warts 15 4. RESULTS 16 4.1. Treatments of scars 16 4.1.1. Treatment of scars in dark skin types 16 4.1.2. Treatment of chickenpox scars 21 4.2. Treatments of warts 24 4.2.1. Treatments of genital warts 24 4.2.2. Treatments of plane warts 27 5. DISCUSSION 29 5.1. Laser treatment of scars 29 5.2. Laser treatment of warts 31 6. -
Human Papillomavirus Infection: Management and Treatment Suchibrata Das
Chapter Human Papillomavirus Infection: Management and Treatment Suchibrata Das Abstract Human papillomavirus infections are very common and recurrent. Their presen- tation varies according to their site of affection. Spontaneous recovery is common in a good number of patients. An ideal wart therapy resolves all or maximum number of warts, is painless, needs only one or a part of a wart treated, needs only minimum number of treatments, leaves no scar, offers lifetime HPV immunity and is easily available for all patients. Various modalities of treatment are available—from some folk and alternative therapies to destructive, antimitotic, virucidal, immunotherapy and combination of therapies. In every modality, the result is significant. Younger individuals with short duration of illness usually have the highest clearance rates for various treatments. Recurrence rate is also high in almost every treatment modality. Immunotherapy has a promising role. Keywords: human papillomavirus infection, chemical cautery, electrocautery, cryotherapy, laser therapy, immunotherapy 1. Introduction Human papillomaviruses (HPVs) are a large and diverse group of viruses with 174 completely characterised types, with new HPV types being continuously found [1]. There are five major HPV genera: Alphapapillomavirus, Betapapillomavirus, Gamma papillomavirus, Mu papillomavirus and Nu papillomavirus. HPVs infect epithelial cells in genital mucosa (Alphapapillomaviruses only), oral mucosa or skin (repre- sentatives of all five genera). The most common clinical manifestation is verruca, with different morphological forms. The histology shows acanthosis, elongation of dermal papillae, presence of vacuolated cells and koilocytes. Subclinical manifesta- tions are invisible to the human eye. These subclinical lesions are flat and multiple. Their clinical insignificance facilitates their spread, and in women their persistence is possibly related to genital cancer.