Progress in L2-Based Prophylactic Vaccine Development for Protection Against Diverse Human Papillomavirus Genotypes and Associated Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Male Reproductive System
Management of Men’s Reproductive 3 Health Problems Men’s Reproductive Health Curriculum Management of Men’s Reproductive 3 Health Problems © 2003 EngenderHealth. All rights reserved. 440 Ninth Avenue New York, NY 10001 U.S.A. Telephone: 212-561-8000 Fax: 212-561-8067 e-mail: [email protected] www.engenderhealth.org This publication was made possible, in part, through support provided by the Office of Population, U.S. Agency for International Development (USAID), under the terms of cooperative agreement HRN-A-00-98-00042-00. The opinions expressed herein are those of the publisher and do not necessarily reflect the views of USAID. Cover design: Virginia Taddoni ISBN 1-885063-45-8 Printed in the United States of America. Printed on recycled paper. Library of Congress Cataloging-in-Publication Data Men’s reproductive health curriculum : management of men’s reproductive health problems. p. ; cm. Companion v. to: Introduction to men’s reproductive health services, and: Counseling and communicating with men. Includes bibliographical references. ISBN 1-885063-45-8 1. Andrology. 2. Human reproduction. 3. Generative organs, Male--Diseases--Treatment. I. EngenderHealth (Firm) II. Counseling and communicating with men. III. Title: Introduction to men’s reproductive health services. [DNLM: 1. Genital Diseases, Male. 2. Physical Examination--methods. 3. Reproductive Health Services. WJ 700 M5483 2003] QP253.M465 2003 616.6’5--dc22 2003063056 Contents Acknowledgments v Introduction vii 1 Disorders of the Male Reproductive System 1.1 The Male -

Changes to Virus Taxonomy 2004
Arch Virol (2005) 150: 189–198 DOI 10.1007/s00705-004-0429-1 Changes to virus taxonomy 2004 M. A. Mayo (ICTV Secretary) Scottish Crop Research Institute, Invergowrie, Dundee, U.K. Received July 30, 2004; accepted September 25, 2004 Published online November 10, 2004 c Springer-Verlag 2004 This note presents a compilation of recent changes to virus taxonomy decided by voting by the ICTV membership following recommendations from the ICTV Executive Committee. The changes are presented in the Table as decisions promoted by the Subcommittees of the EC and are grouped according to the major hosts of the viruses involved. These new taxa will be presented in more detail in the 8th ICTV Report scheduled to be published near the end of 2004 (Fauquet et al., 2004). Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., and Ball, L.A. (eds) (2004). Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press, London, pp. 1258. Recent changes to virus taxonomy Viruses of vertebrates Family Arenaviridae • Designate Cupixi virus as a species in the genus Arenavirus • Designate Bear Canyon virus as a species in the genus Arenavirus • Designate Allpahuayo virus as a species in the genus Arenavirus Family Birnaviridae • Assign Blotched snakehead virus as an unassigned species in family Birnaviridae Family Circoviridae • Create a new genus (Anellovirus) with Torque teno virus as type species Family Coronaviridae • Recognize a new species Severe acute respiratory syndrome coronavirus in the genus Coro- navirus, family Coronaviridae, order Nidovirales -

Comparative Analysis, Distribution, and Characterization of Microsatellites in Orf Virus Genome
www.nature.com/scientificreports OPEN Comparative analysis, distribution, and characterization of microsatellites in Orf virus genome Basanta Pravas Sahu1, Prativa Majee 1, Ravi Raj Singh1, Anjan Sahoo2 & Debasis Nayak 1* Genome-wide in-silico identifcation of microsatellites or simple sequence repeats (SSRs) in the Orf virus (ORFV), the causative agent of contagious ecthyma has been carried out to investigate the type, distribution and its potential role in the genome evolution. We have investigated eleven ORFV strains, which resulted in the presence of 1,036–1,181 microsatellites per strain. The further screening revealed the presence of 83–107 compound SSRs (cSSRs) per genome. Our analysis indicates the dinucleotide (76.9%) repeats to be the most abundant, followed by trinucleotide (17.7%), mononucleotide (4.9%), tetranucleotide (0.4%) and hexanucleotide (0.2%) repeats. The Relative Abundance (RA) and Relative Density (RD) of these SSRs varied between 7.6–8.4 and 53.0–59.5 bp/ kb, respectively. While in the case of cSSRs, the RA and RD ranged from 0.6–0.8 and 12.1–17.0 bp/kb, respectively. Regression analysis of all parameters like the incident of SSRs, RA, and RD signifcantly correlated with the GC content. But in a case of genome size, except incident SSRs, all other parameters were non-signifcantly correlated. Nearly all cSSRs were composed of two microsatellites, which showed no biasedness to a particular motif. Motif duplication pattern, such as, (C)-x-(C), (TG)- x-(TG), (AT)-x-(AT), (TC)- x-(TC) and self-complementary motifs, such as (GC)-x-(CG), (TC)-x-(AG), (GT)-x-(CA) and (TC)-x-(AG) were observed in the cSSRs. -
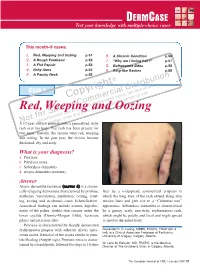
Red, Weeping and Oozing P.51 6
DERM CASE Test your knowledge with multiple-choice cases This month–9 cases: 1. Red, Weeping and Oozing p.51 6. A Chronic Condition p.56 2. A Rough Forehead p.52 7. “Why am I losing hair?” p.57 3. A Flat Papule p.53 8. Bothersome Bites p.58 4. Itchy Arms p.54 9. Ring-like Rashes p.59 5. A Patchy Neck p.55 on © buti t ri , h ist oad rig D wnl Case 1 y al n do p ci ca use o er sers nal C m d u rso m rise r pe o utho y fo C d. A cop or bite ngle Red, Weleepirnohig a sind Oozing a se p rint r S ed u nd p o oris w a t f uth , vie o Una lay AN12-year-old boy dpriesspents with a generalized, itchy rash over his body. The rash has been present for two years. Initially, the lesions were red, weeping and oozing. In the past year, the lesions became thickened, dry and scaly. What is your diagnosis? a. Psoriasis b. Pityriasis rosea c. Seborrheic dermatitis d. Atopic dermatitis (eczema) Answer Atopic dermatitis (eczema) (answer d) is a chroni - cally relapsing dermatosis characterized by pruritus, later by a widespread, symmetrical eruption in erythema, vesiculation, papulation, oozing, crust - which the long axes of the rash extend along skin ing, scaling and, in chronic cases, lichenification. tension lines and give rise to a “Christmas tree” Associated findings can include xerosis, hyperlin - appearance. Seborrheic dermatitis is characterized earity of the palms, double skin creases under the by a greasy, scaly, non-itchy, erythematous rash, lower eyelids (Dennie-Morgan folds), keratosis which might be patchy and focal and might spread pilaris and pityriasis alba. -

The P1N-PISPO Trans-Frame Gene of Sweet Potato Feathery Mottle
crossmark The P1N-PISPO trans-Frame Gene of Sweet Potato Feathery Mottle Potyvirus Is Produced during Virus Infection and Functions as an RNA Silencing Suppressor Downloaded from Ares Mingot,a Adrián Valli,b Bernardo Rodamilans,c David San León,c David C. Baulcombe,b Juan Antonio García,c Juan José López-Moyaa Center for Research in Agricultural Genomics, CSIC-IRTA-UAB-UB, Cerdanyola del Vallès, Barcelona, Spaina; Department of Plant Sciences, University of Cambridge, Cambridge, United Kingdomb; Centro Nacional de Biotecnología CNB, CSIC, Madrid, Spainc ABSTRACT The positive-sense RNA genome of Sweet potato feathery mottle virus (SPFMV) (genus Potyvirus, family Potyviridae) contains a /large open reading frame (ORF) of 3,494 codons translatable as a polyprotein and two embedded shorter ORFs in the ؊1 frame: http://jvi.asm.org PISPO, of 230 codons, and PIPO, of 66 codons, located in the P1 and P3 regions, respectively. PISPO is specific to some sweet potato-infecting potyviruses, while PIPO is present in all potyvirids. In SPFMV these two extra ORFs are preceded by conserved G2A6 motifs. We have shown recently that a polymerase slippage mechanism at these sites could produce transcripts bringing these ORFs in frame with the upstream polyprotein, thus leading to P1N-PISPO and P3N-PIPO products (B. Rodamilans, A. Valli, A. Mingot, D. San Leon, D. B. Baulcombe, J. J. Lopez-Moya, and J.A. Garcia, J Virol 89:6965–6967, 2015, doi:10.1128/JVI.00 337-15). Here, we demonstrate by liquid chromatography coupled to mass spectrometry that both P1 and P1N-PISPO are produced during viral infection and coexist in SPFMV-infected Ipomoea batatas plants. -

Virus World As an Evolutionary Network of Viruses and Capsidless Selfish Elements
Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Koonin, E. V., & Dolja, V. V. (2014). Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements. Microbiology and Molecular Biology Reviews, 78(2), 278-303. doi:10.1128/MMBR.00049-13 10.1128/MMBR.00049-13 American Society for Microbiology Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Eugene V. Koonin,a Valerian V. Doljab National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland, USAa; Department of Botany and Plant Pathology and Center for Genome Research and Biocomputing, Oregon State University, Corvallis, Oregon, USAb Downloaded from SUMMARY ..................................................................................................................................................278 INTRODUCTION ............................................................................................................................................278 PREVALENCE OF REPLICATION SYSTEM COMPONENTS COMPARED TO CAPSID PROTEINS AMONG VIRUS HALLMARK GENES.......................279 CLASSIFICATION OF VIRUSES BY REPLICATION-EXPRESSION STRATEGY: TYPICAL VIRUSES AND CAPSIDLESS FORMS ................................279 EVOLUTIONARY RELATIONSHIPS BETWEEN VIRUSES AND CAPSIDLESS VIRUS-LIKE GENETIC ELEMENTS ..............................................280 Capsidless Derivatives of Positive-Strand RNA Viruses....................................................................................................280 -

Aphid Transmission of Potyvirus: the Largest Plant-Infecting RNA Virus Genus
Supplementary Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus Kiran R. Gadhave 1,2,*,†, Saurabh Gautam 3,†, David A. Rasmussen 2 and Rajagopalbabu Srinivasan 3 1 Department of Plant Pathology and Microbiology, University of California, Riverside, CA 92521, USA 2 Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC 27606, USA; [email protected] 3 Department of Entomology, University of Georgia, 1109 Experiment Street, Griffin, GA 30223, USA; [email protected] * Correspondence: [email protected]. † Authors contributed equally. Received: 13 May 2020; Accepted: 15 July 2020; Published: date Abstract: Potyviruses are the largest group of plant infecting RNA viruses that cause significant losses in a wide range of crops across the globe. The majority of viruses in the genus Potyvirus are transmitted by aphids in a non-persistent, non-circulative manner and have been extensively studied vis-à-vis their structure, taxonomy, evolution, diagnosis, transmission and molecular interactions with hosts. This comprehensive review exclusively discusses potyviruses and their transmission by aphid vectors, specifically in the light of several virus, aphid and plant factors, and how their interplay influences potyviral binding in aphids, aphid behavior and fitness, host plant biochemistry, virus epidemics, and transmission bottlenecks. We present the heatmap of the global distribution of potyvirus species, variation in the potyviral coat protein gene, and top aphid vectors of potyviruses. Lastly, we examine how the fundamental understanding of these multi-partite interactions through multi-omics approaches is already contributing to, and can have future implications for, devising effective and sustainable management strategies against aphid- transmitted potyviruses to global agriculture. -

Activation of Flat Warts (Verrucae Planae) on the Q-Switched Laser-Assisted Tattoo Removal Site
CR(Case Report) Med Laser 2014;3(2):84-86 pISSN 2287-8300ㆍeISSN 2288-0224 Activation of Flat Warts (Verrucae Planae) on the Q-Switched Laser-Assisted Tattoo Removal Site Nark-kyoung Rho Flat warts, or verrucae planae, are a common cutaneous infection, which tends to be multiple and grouped. They are more often found on sun- exposed areas such as the face, neck, and extremities, and sometimes Leaders Aesthetic Laser and Cosmetic Surgery develop at the sites of skin trauma. The author reports on a case of flat Center, Seoul, Korea warts which developed on the Q-switched laser tattoo removal site. Histologic findings confirmed the diagnosis of verruca plana. The author suggests that activation of the human papillomavirus should be included in the possible complications of the laser-assisted tattoo removal procedure. Key words Human papillomavirus; Q-switched lasers; Tattooing; Warts Received November 28, 2014 Revised December 3, 2014 Accepted December 4, 2014 Correspondence Nark-kyoung Rho Leaders Aesthetic Laser and Cosmetic Surgery Center, THE CLASSIC500, 90, Neungdong-ro, Gwangjin-gu, Seoul 143-854, Korea Tel: +82-2-444-7585 Fax: +82-2-444-7535 E-mail: [email protected] C Korean Society for Laser Medicine and Surgery CC This is an open access article distributed under the terms of the Creative Commons Attribution Non- Commercial License (http://creativecommons.org/ licenses/by-nc/3.0) which permits unrestricted non- commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. 84 Medical Lasers; Engineering, Basic Research, and Clinical Application Warts on the Laser Tattoo Removal Site Nark-kyoung Rho Case Report INTRODUCTION mm spot size, and fluence of 12 J/cm2. -

Human Papillomavirus and Related Diseases – from Bench to Bedside
HUMAN PAPILLOMAVIRUS AND RELATED DISEASES – FROM BENCH TO BEDSIDE A CLINICAL PERSPECTIVE Edited by Davy Vanden Broeck Human Papillomavirus and Related Diseases – From Bench to Bedside – A Clinical Perspective Edited by Davy Vanden Broeck Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2011 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. Any republication, referencing or personal use of the work must explicitly identify the original source. As for readers, this license allows users to download, copy and build upon published chapters even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. Notice Statements and opinions expressed in the chapters are these of the individual contributors and not necessarily those of the editors or publisher. No responsibility is accepted for the accuracy of information contained in the published chapters. The publisher assumes no responsibility for any damage or injury to persons or property arising out of the use of any materials, instructions, methods or ideas contained in the book. Publishing Process Manager Ivona Lovric Technical Editor Teodora Smiljanic Cover Designer InTech Design Team First published January, 2012 Printed in Croatia A free online edition of this book is available at www.intechopen.com Additional hard copies can be obtained from [email protected] Human Papillomavirus and Related Diseases – From Bench to Bedside – A Clinical Perspective, Edited by Davy Vanden Broeck p. -

Human Papillomavirus Infection in Child G
The Open Dermatology Journal, 2009, 3, 111-116 111 Open Access Human Papillomavirus Infection in Child G. Fabbrocini*, S. Cacciapuoti and G. Monfrecola Department of Systematic Pathology, Section of Dermatology, University of Naples Federico II, Naples, Italy Abstract: Human papilloma viruses (HPV) have been identified as the cause of cutaneous and genital warts. Furthermore, HPV DNA can also be detected in certain malignant epithelial tumors such as cervical carcinoma and cutaneous squamous cell cancer. HPV infections in children, particularly when occurring as condylomata acuminata, present a difficult and often puzzling problem. The modes of viral transmission in child remain controversial, including perinatal transmission, auto- and hetero-inoculation, sexual abuse, and, possibly, indirect transmission via fomites. The treatment of warts and condylomata acuminate in child poses a therapeutic challenge for physicians. No single therapy has been proven effective at achieving complete remission in every patient. As a result, many different approaches to therapy exist. The proper approach to the management of warts depends on the age of the patient, location, size, extent, and type of wart, and duration of lesions. Each treatment decision should be made on a case-by-case basis according to the experience of the physician, patient preference, and the application of evidence-based medicine. In order to modify HPV epidemiology, HPV prophylactic vaccine has been recently proposed for children. The purpose of this review is to update the reader with the latest information on the HPV and its therapeutics in children. Keywords: HPV, child, perinatal transmission, sexual abuse. INTRODUCTION because they have not yet been propagated in tissue culture. -

Molecular Analysis of Vanilla Mosaic Virus from the Cook Islands
Molecular Analysis of Vanilla mosaic virus from the Cook Islands Christopher Puli’uvea A thesis submitted to Auckland University of Technology in partial fulfilment of the requirements for the degree of Master of Science (MSc) 2017 School of Science I Abstract Vanilla was first introduced to French Polynesia in 1848 and from 1899-1966 was a major export for French Polynesia who then produced an average of 158 tonnes of cured Vanilla tahitensis beans annually. In 1967, vanilla production declined rapidly to a low of 0.6 tonnes by 1981, which prompted a nation-wide investigation with the aim of restoring vanilla production to its former levels. As a result, a mosaic-inducing virus was discovered infecting V. tahitensis that was distinct from Cymbidium mosaic virus (CyMV) and Odontoglossum ringspot virus (ORSV) but serologically related to dasheen mosaic virus (DsMV). The potyvirus was subsequently named vanilla mosaic virus (VanMV) and was later reported to infect V. tahitensis in the Cook Islands and V. planifolia in Fiji and Vanuatu. Attempts were made to mechanically inoculate VanMV to a number of plants that are susceptible to DsMV, but with no success. Based on a partial sequence analysis, VanMV-FP (French Polynesian isolate) and VanMV-CI (Cook Islands isolate) were later characterised as strains of DsMV exclusively infecting vanilla. Since its discovery, little information is known about how VanMV-CI acquired the ability to exclusively infect vanilla and lose its ability to infect natural hosts of DsMV or vice versa. The aims of this research were to characterise the VanMV genome and attempt to determine the molecular basis for host range specificity of VanMV-CI. -

Characterization of a New Potyvirus Causing Mosaic and Flower Variegation in Catharanthus Roseus in Brazil
New potyvirus causing mosaic and fl ower variegation in C. roseus 687 Note Characterization of a new potyvirus causing mosaic and fl ower variegation in Catharanthus roseus in Brazil Sheila Conceição Maciel1, Ricardo Ferreira da Silva1, Marcelo Silva Reis2, Adriana Salomão Jadão3, Daniel Dias Rosa1, José Segundo Giampan4, Elliot Watanabe Kitajima3, Jorge Alberto Marques Rezende3*, Luis Eduardo Aranha Camargo3 1USP/ESALQ – Programa de Pós-Graduação em Fitopatologia. 2Centro de Citricultura Sylvio Moreira/Bioinformática – 13490-970 – Cordeirópolis, SP – Brasil. 3USP/ESALQ – Depto. de Fitopatologia e Nematologia – C.P. 09 – 13418-900 – Piracicaba, SP – Brasil. 4IAPAR – Área de Proteção de Plantas – C.P. 481 - 86047-902 – Londrina, PR – Brasil. *Corresponding author <[email protected]> Edited by: Luís Reynaldo Ferracciú Alleoni ABSTRACT: Catharanthus roseus is a perennial, evergreen herb in the family Apocynaceae, which is used as ornamental and for popular medicine to treat a wide assortment of human diseases. This paper describes a new potyvirus found causing mosaic symptom, foliar malformation and flower variegation in C. roseus. Of 28 test-plants inoculated mechanically with this potyvirus, only C. roseus and Nicotiana benthamiana developed systemic mosaic, whereas Chenopodium amaranticolor and C. quinoa exhibited chlorotic local lesions. The virus was transmitted by Aphis gossypii and Myzus nicotianae. When the nucleotide sequence of the CP gene (768nt) was compared with other members of the Potyviridae family, the highest identities varied from 67 to 76 %. For the 3' UTR (286nt), identities varied from 16.8 to 28.6 %. The name Catharanthus mosaic virus (CatMV) is proposed for this new potyvirus. Keywords: RT-PCR, Apocynaceae, periwinkle, diagnose, genome sequencing Introduction Cucumis sativus, Cucurbita pepo cv.