THE CONCISE GUIDE to PHARMACOLOGY 2017/18: Catalytic Receptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Immunogenic Cell Death
University of Southern Denmark Current approaches for combination therapy of cancer The role of immunogenic cell death Asadzadeh, Zahra; Safarzadeh, Elham; Safaei, Sahar; Baradaran, Ali; Mohammadi, Ali; Hajiasgharzadeh, Khalil; Derakhshani, Afshin; Argentiero, Antonella; Silvestris, Nicola; Baradaran, Behzad Published in: Cancers DOI: 10.3390/cancers12041047 Publication date: 2020 Document version: Final published version Document license: CC BY Citation for pulished version (APA): Asadzadeh, Z., Safarzadeh, E., Safaei, S., Baradaran, A., Mohammadi, A., Hajiasgharzadeh, K., Derakhshani, A., Argentiero, A., Silvestris, N., & Baradaran, B. (2020). Current approaches for combination therapy of cancer: The role of immunogenic cell death. Cancers , 12(4), [1047]. https://doi.org/10.3390/cancers12041047 Go to publication entry in University of Southern Denmark's Research Portal Terms of use This work is brought to you by the University of Southern Denmark. Unless otherwise specified it has been shared according to the terms for self-archiving. If no other license is stated, these terms apply: • You may download this work for personal use only. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying this open access version If you believe that this document breaches copyright please contact us providing details and we will investigate your claim. Please direct all enquiries to [email protected] Download date: 04. Oct. 2021 cancers Review Current -

Cytokine Nomenclature
RayBiotech, Inc. The protein array pioneer company Cytokine Nomenclature Cytokine Name Official Full Name Genbank Related Names Symbol 4-1BB TNFRSF Tumor necrosis factor NP_001552 CD137, ILA, 4-1BB ligand receptor 9 receptor superfamily .2. member 9 6Ckine CCL21 6-Cysteine Chemokine NM_002989 Small-inducible cytokine A21, Beta chemokine exodus-2, Secondary lymphoid-tissue chemokine, SLC, SCYA21 ACE ACE Angiotensin-converting NP_000780 CD143, DCP, DCP1 enzyme .1. NP_690043 .1. ACE-2 ACE2 Angiotensin-converting NP_068576 ACE-related carboxypeptidase, enzyme 2 .1 Angiotensin-converting enzyme homolog ACTH ACTH Adrenocorticotropic NP_000930 POMC, Pro-opiomelanocortin, hormone .1. Corticotropin-lipotropin, NPP, NP_001030 Melanotropin gamma, Gamma- 333.1 MSH, Potential peptide, Corticotropin, Melanotropin alpha, Alpha-MSH, Corticotropin-like intermediary peptide, CLIP, Lipotropin beta, Beta-LPH, Lipotropin gamma, Gamma-LPH, Melanotropin beta, Beta-MSH, Beta-endorphin, Met-enkephalin ACTHR ACTHR Adrenocorticotropic NP_000520 Melanocortin receptor 2, MC2-R hormone receptor .1 Activin A INHBA Activin A NM_002192 Activin beta-A chain, Erythroid differentiation protein, EDF, INHBA Activin B INHBB Activin B NM_002193 Inhibin beta B chain, Activin beta-B chain Activin C INHBC Activin C NM005538 Inhibin, beta C Activin RIA ACVR1 Activin receptor type-1 NM_001105 Activin receptor type I, ACTR-I, Serine/threonine-protein kinase receptor R1, SKR1, Activin receptor-like kinase 2, ALK-2, TGF-B superfamily receptor type I, TSR-I, ACVRLK2 Activin RIB ACVR1B -

Katalog 2015 Cover Paul Lin *Hinweis Förderung.Indd
Product List 2015 WE LIVE SERVICE Certificates quartett owns two productions sites that are certified according to EN ISO 9001:2008 Quality management systems - Requirements EN ISO 13485:2012 + AC:2012 Medical devices - Quality management systems - Requirements for regulatory purposes GMP Conformity Our quality management guarantees products of highest quality! 2 Foreword to the quartett product list 2015 quartett Immunodiagnostika, Biotechnologie + Kosmetik Vertriebs GmbH welcomes you as one of our new business partners as well as all of our previous loyal clients. You are now member of quartett´s worldwide customers. First of all we would like to introduce ourselves to you. Founded as a family-run company in 1986, quartett ensures for more than a quarter of a century consistent quality of products. Service and support of our valued customers are our daily businesses. And we will continue! In the end 80´s quartett offered radioimmunoassay and enzyme immunoassay kits from different manufacturers in the USA. In the beginning 90´s the company changed its strategy from offering products for routine diagnostic to the increasing field of research and development. Setting up a production plant in 1997 and a second one in 2011 supported this decision. The company specialized its product profile in the field of manufacturing synthetic peptides for antibody production, peptides such as protease inhibitors, biochemical reagents and products for histology, cytology and immunohistology. All products are exclusively manufactured in Germany without outsourcing any production step. Nowadays, we expand into all other diagnostic and research fields and supply our customers in universities, government institutes, pharmaceutical and biotechnological companies, hospitals, and private doctor offices. -

Supporting Online Material
1 2 3 4 5 6 7 Supplementary Information for 8 9 Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic 10 retinopathy 11 12 *Samuel A. Mills, *Andrew I. Jobling, *Michael A. Dixon, Bang V. Bui, Kirstan A. Vessey, Joanna A. Phipps, 13 Ursula Greferath, Gene Venables, Vickie H.Y. Wong, Connie H.Y. Wong, Zheng He, Flora Hui, James C. 14 Young, Josh Tonc, Elena Ivanova, Botir T. Sagdullaev, Erica L. Fletcher 15 * Joint first authors 16 17 Corresponding author: 18 Prof. Erica L. Fletcher. Department of Anatomy & Neuroscience. The University of Melbourne, Grattan St, 19 Parkville 3010, Victoria, Australia. 20 Email: [email protected] ; Tel: +61-3-8344-3218; Fax: +61-3-9347-5219 21 22 This PDF file includes: 23 24 Supplementary text 25 Figures S1 to S10 26 Tables S1 to S7 27 Legends for Movies S1 to S2 28 SI References 29 30 Other supplementary materials for this manuscript include the following: 31 32 Movies S1 to S2 33 34 35 36 1 1 Supplementary Information Text 2 Materials and Methods 3 Microglial process movement on retinal vessels 4 Dark agouti rats were anaesthetized, injected intraperitoneally with rhodamine B (Sigma-Aldrich) to label blood 5 vessels and retinal explants established as described in the main text. Retinal microglia were labelled with Iba-1 6 and imaging performed on an inverted confocal microscope (Leica SP5). Baseline images were taken for 10 7 minutes, followed by the addition of PBS (10 minutes) and then either fractalkine or fractalkine + candesartan 8 (10 minutes) using concentrations outlined in the main text. -

TNF Decoy Receptors Encoded by Poxviruses
pathogens Review TNF Decoy Receptors Encoded by Poxviruses Francisco Javier Alvarez-de Miranda † , Isabel Alonso-Sánchez † , Antonio Alcamí and Bruno Hernaez * Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas, Campus de Cantoblanco, Universidad Autónoma de Madrid, Nicolás Cabrera 1, 28049 Madrid, Spain; [email protected] (F.J.A.-d.M.); [email protected] (I.A.-S.); [email protected] (A.A.) * Correspondence: [email protected]; Tel.: +34-911-196-4590 † These authors contributed equally. Abstract: Tumour necrosis factor (TNF) is an inflammatory cytokine produced in response to viral infections that promotes the recruitment and activation of leukocytes to sites of infection. This TNF- based host response is essential to limit virus spreading, thus poxviruses have evolutionarily adopted diverse molecular mechanisms to counteract TNF antiviral action. These include the expression of poxvirus-encoded soluble receptors or proteins able to bind and neutralize TNF and other members of the TNF ligand superfamily, acting as decoy receptors. This article reviews in detail the various TNF decoy receptors identified to date in the genomes from different poxvirus species, with a special focus on their impact on poxvirus pathogenesis and their potential use as therapeutic molecules. Keywords: poxvirus; immune evasion; tumour necrosis factor; tumour necrosis factor receptors; lymphotoxin; inflammation; cytokines; secreted decoy receptors; vaccinia virus; ectromelia virus; cowpox virus Citation: Alvarez-de Miranda, F.J.; Alonso-Sánchez, I.; Alcamí, A.; 1. TNF Biology Hernaez, B. TNF Decoy Receptors TNF is a potent pro-inflammatory cytokine with a broad range of biological effects, Encoded by Poxviruses. Pathogens ranging from the activation of inflammatory gene programs to cell differentiation or 2021, 10, 1065. -

To Study Mutant P53 Gain of Function, Various Tumor-Derived P53 Mutants
Differential effects of mutant TAp63γ on transactivation of p53 and/or p63 responsive genes and their effects on global gene expression. A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Shama K Khokhar M.Sc., Bilaspur University, 2004 B.Sc., Bhopal University, 2002 2007 1 COPYRIGHT SHAMA K KHOKHAR 2007 2 WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES Date of Defense: 12-03-07 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY SHAMA KHAN KHOKHAR ENTITLED Differential effects of mutant TAp63γ on transactivation of p53 and/or p63 responsive genes and their effects on global gene expression BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science Madhavi P. Kadakia, Ph.D. Thesis Director Daniel Organisciak , Ph.D. Department Chair Committee on Final Examination Madhavi P. Kadakia, Ph.D. Steven J. Berberich, Ph.D. Michael Leffak, Ph.D. Joseph F. Thomas, Jr., Ph.D. Dean, School of Graduate Studies 3 Abstract Khokhar, Shama K. M.S., Department of Biochemistry and Molecular Biology, Wright State University, 2007 Differential effect of TAp63γ mutants on transactivation of p53 and/or p63 responsive genes and their effects on global gene expression. p63, a member of the p53 gene family, known to play a role in development, has more recently also been implicated in cancer progression. Mice lacking p63 exhibit severe developmental defects such as limb truncations, abnormal skin, and absence of hair follicles, teeth, and mammary glands. Germline missense mutations of p63 have been shown to be responsible for several human developmental syndromes including SHFM, EEC and ADULT syndromes and are associated with anomalies in the development of organs of epithelial origin. -

Supplemental Materials ZNF281 Enhances Cardiac Reprogramming
Supplemental Materials ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression Huanyu Zhou, Maria Gabriela Morales, Hisayuki Hashimoto, Matthew E. Dickson, Kunhua Song, Wenduo Ye, Min S. Kim, Hanspeter Niederstrasser, Zhaoning Wang, Beibei Chen, Bruce A. Posner, Rhonda Bassel-Duby and Eric N. Olson Supplemental Table 1; related to Figure 1. Supplemental Table 2; related to Figure 1. Supplemental Table 3; related to the “quantitative mRNA measurement” in Materials and Methods section. Supplemental Table 4; related to the “ChIP-seq, gene ontology and pathway analysis” and “RNA-seq” and gene ontology analysis” in Materials and Methods section. Supplemental Figure S1; related to Figure 1. Supplemental Figure S2; related to Figure 2. Supplemental Figure S3; related to Figure 3. Supplemental Figure S4; related to Figure 4. Supplemental Figure S5; related to Figure 6. Supplemental Table S1. Genes included in human retroviral ORF cDNA library. Gene Gene Gene Gene Gene Gene Gene Gene Symbol Symbol Symbol Symbol Symbol Symbol Symbol Symbol AATF BMP8A CEBPE CTNNB1 ESR2 GDF3 HOXA5 IL17D ADIPOQ BRPF1 CEBPG CUX1 ESRRA GDF6 HOXA6 IL17F ADNP BRPF3 CERS1 CX3CL1 ETS1 GIN1 HOXA7 IL18 AEBP1 BUD31 CERS2 CXCL10 ETS2 GLIS3 HOXB1 IL19 AFF4 C17ORF77 CERS4 CXCL11 ETV3 GMEB1 HOXB13 IL1A AHR C1QTNF4 CFL2 CXCL12 ETV7 GPBP1 HOXB5 IL1B AIMP1 C21ORF66 CHIA CXCL13 FAM3B GPER HOXB6 IL1F3 ALS2CR8 CBFA2T2 CIR1 CXCL14 FAM3D GPI HOXB7 IL1F5 ALX1 CBFA2T3 CITED1 CXCL16 FASLG GREM1 HOXB9 IL1F6 ARGFX CBFB CITED2 CXCL3 FBLN1 GREM2 HOXC4 IL1F7 -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Angiopoietin 4 (ANGPT4) (NM 015985) Human Untagged Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for SC304373 Angiopoietin 4 (ANGPT4) (NM_015985) Human Untagged Clone Product data: Product Type: Expression Plasmids Product Name: Angiopoietin 4 (ANGPT4) (NM_015985) Human Untagged Clone Tag: Tag Free Symbol: ANGPT4 Synonyms: ANG3; ANG4 Vector: pCMV6-XL5 E. coli Selection: Ampicillin (100 ug/mL) Cell Selection: None This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 3 Angiopoietin 4 (ANGPT4) (NM_015985) Human Untagged Clone – SC304373 Fully Sequenced ORF: >OriGene sequence for NM_015985 edited CAGGCAAGCCTGGCCACTGTTGGCTGCAGCAGGACATCCCAGGCACAGCCCCTAGGGCTC TGAGCAGACATCCCTCGCCATTGACACATCTTCAGATGCTCTCCCAGCTAGCCATGCTGC AGGGCAGCCTCCTCCTTGTGGTTGCCACCATGTCTGTGGCTCAACAGACAAGGCAGGAGG CGGATAGGGGCTGCGAGACACTTGTAGTCCAGCACGGCCACTGTAGCTACACCTTCTTGC TGCCCAAGTCTGAGCCCTGCCCTCCGGGGCCTGAGGTCTCCAGGGACTCCAACACCCTCC AGAGAGAATCACTGGCCAACCCACTGCACCTGGGGAAGTTGCCCACCCAGCAGGTGAAAC AGCTGGAGCAGGCACTGCAGAACAACACGCAGTGGCTGAAGAAGCTAGAGAGGGCCATCA AGACGATCTTGAGGTCGAAGCTGGAGCAGGTCCAGCAGCAAATGGCCCAGAATCAGACGG CCCCCATGCTAGAGCTGGGCACCAGCCTCCTGAACCAGACCACTGCCCAGATCCGCAAGC TGACCGACATGGAGGCTCAGCTCCTGAACCAGACATCAAGAATGGATGCCCAGATGCCAG AGACCTTTCTGTCCACCAACAAGCTGGAGAACCAGCTGCTGCTACAGAGGCAGAAGCTCC AGCAGCTTCAGGGCCAAAACAGCGCGCTCGAGAAGCGGTTGCAGGCCCTGGAGACCAAGC -
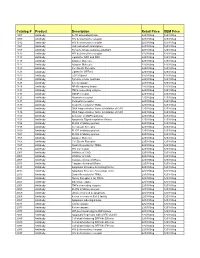
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

Jimmunol.0903453.Full.Pdf
Antiviral Response Dictated by Choreographed Cascade of Transcription Factors This information is current as Elena Zaslavsky, Uri Hershberg, Jeremy Seto, Alissa M. of October 2, 2021. Pham, Susanna Marquez, Jamie L. Duke, James G. Wetmur, Benjamin R. tenOever, Stuart C. Sealfon and Steven H. Kleinstein J Immunol published online 17 February 2010 http://www.jimmunol.org/content/early/2010/02/17/jimmun ol.0903453 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2010/02/15/jimmunol.090345 Material 3.DC1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 2, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published February 17, 2010, doi:10.4049/jimmunol.0903453 The Journal of Immunology Antiviral Response Dictated by Choreographed Cascade of Transcription Factors Elena Zaslavsky,*,†,1 Uri Hershberg,‡,1 Jeremy Seto,*,† Alissa M. Pham,x Susanna Marquez,‡ Jamie L. Duke,{ James G. Wetmur,x Benjamin R. tenOever,x Stuart C. Sealfon,*,† and Steven H. -

1714 Gene Comprehensive Cancer Panel Enriched for Clinically Actionable Genes with Additional Biologically Relevant Genes 400-500X Average Coverage on Tumor
xO GENE PANEL 1714 gene comprehensive cancer panel enriched for clinically actionable genes with additional biologically relevant genes 400-500x average coverage on tumor Genes A-C Genes D-F Genes G-I Genes J-L AATK ATAD2B BTG1 CDH7 CREM DACH1 EPHA1 FES G6PC3 HGF IL18RAP JADE1 LMO1 ABCA1 ATF1 BTG2 CDK1 CRHR1 DACH2 EPHA2 FEV G6PD HIF1A IL1R1 JAK1 LMO2 ABCB1 ATM BTG3 CDK10 CRK DAXX EPHA3 FGF1 GAB1 HIF1AN IL1R2 JAK2 LMO7 ABCB11 ATR BTK CDK11A CRKL DBH EPHA4 FGF10 GAB2 HIST1H1E IL1RAP JAK3 LMTK2 ABCB4 ATRX BTRC CDK11B CRLF2 DCC EPHA5 FGF11 GABPA HIST1H3B IL20RA JARID2 LMTK3 ABCC1 AURKA BUB1 CDK12 CRTC1 DCUN1D1 EPHA6 FGF12 GALNT12 HIST1H4E IL20RB JAZF1 LPHN2 ABCC2 AURKB BUB1B CDK13 CRTC2 DCUN1D2 EPHA7 FGF13 GATA1 HLA-A IL21R JMJD1C LPHN3 ABCG1 AURKC BUB3 CDK14 CRTC3 DDB2 EPHA8 FGF14 GATA2 HLA-B IL22RA1 JMJD4 LPP ABCG2 AXIN1 C11orf30 CDK15 CSF1 DDIT3 EPHB1 FGF16 GATA3 HLF IL22RA2 JMJD6 LRP1B ABI1 AXIN2 CACNA1C CDK16 CSF1R DDR1 EPHB2 FGF17 GATA5 HLTF IL23R JMJD7 LRP5 ABL1 AXL CACNA1S CDK17 CSF2RA DDR2 EPHB3 FGF18 GATA6 HMGA1 IL2RA JMJD8 LRP6 ABL2 B2M CACNB2 CDK18 CSF2RB DDX3X EPHB4 FGF19 GDNF HMGA2 IL2RB JUN LRRK2 ACE BABAM1 CADM2 CDK19 CSF3R DDX5 EPHB6 FGF2 GFI1 HMGCR IL2RG JUNB LSM1 ACSL6 BACH1 CALR CDK2 CSK DDX6 EPOR FGF20 GFI1B HNF1A IL3 JUND LTK ACTA2 BACH2 CAMTA1 CDK20 CSNK1D DEK ERBB2 FGF21 GFRA4 HNF1B IL3RA JUP LYL1 ACTC1 BAG4 CAPRIN2 CDK3 CSNK1E DHFR ERBB3 FGF22 GGCX HNRNPA3 IL4R KAT2A LYN ACVR1 BAI3 CARD10 CDK4 CTCF DHH ERBB4 FGF23 GHR HOXA10 IL5RA KAT2B LZTR1 ACVR1B BAP1 CARD11 CDK5 CTCFL DIAPH1 ERCC1 FGF3 GID4 HOXA11 IL6R KAT5 ACVR2A